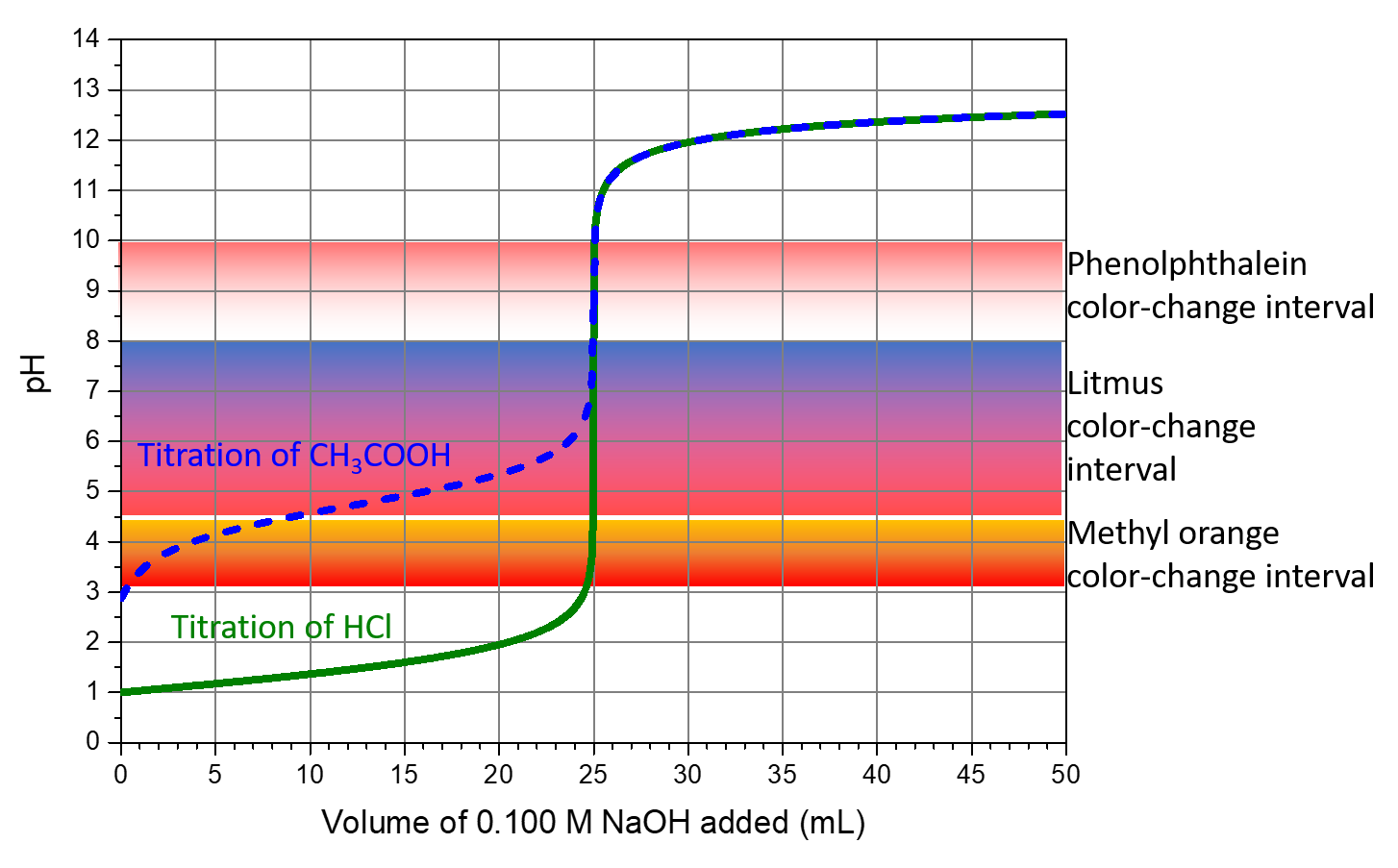
Indicator In A Titration . Find examples of titration experiments and tips to improve your results. Both indicators change colour over a specific ph range. This page assumes that you know about ph curves for all the. An indicator is a chemical that changes color when a titration reaches its endpoint. Demonstrate how to select the proper indicator for a titration experiment; Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. Determine the acidic dissociation constants k a or k ai of indicators. In the strong acid titration,.
from wisc.pb.unizin.org
An indicator is a chemical that changes color when a titration reaches its endpoint. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. Find examples of titration experiments and tips to improve your results. Determine the acidic dissociation constants k a or k ai of indicators. In the strong acid titration,. This page assumes that you know about ph curves for all the. Demonstrate how to select the proper indicator for a titration experiment; Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Both indicators change colour over a specific ph range.
D30.1 AcidBase Indicators Chemistry 109 Fall 2021
Indicator In A Titration Demonstrate how to select the proper indicator for a titration experiment; Find examples of titration experiments and tips to improve your results. An indicator is a chemical that changes color when a titration reaches its endpoint. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. Demonstrate how to select the proper indicator for a titration experiment; In the strong acid titration,. Determine the acidic dissociation constants k a or k ai of indicators. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. This page assumes that you know about ph curves for all the. Both indicators change colour over a specific ph range.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Atoms First Indicator In A Titration Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. In the strong acid titration,. Find examples of titration experiments and tips to improve your results. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. This page assumes that you know about ph curves for all. Indicator In A Titration.
From www.alamy.com
Phenolphthalein is used as a single indicator in acidbase titrations Indicator In A Titration This page assumes that you know about ph curves for all the. Both indicators change colour over a specific ph range. Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The two most common indicators that. Indicator In A Titration.
From www.studypool.com
SOLUTION Acid base indicators titration curves Studypool Indicator In A Titration This page assumes that you know about ph curves for all the. Determine the acidic dissociation constants k a or k ai of indicators. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. Find examples of titration experiments and tips to improve your results. Demonstrate how to select the proper indicator for. Indicator In A Titration.
From studylib.net
The titration of an Indicator (HIn) Indicator In A Titration In the strong acid titration,. An indicator is a chemical that changes color when a titration reaches its endpoint. Determine the acidic dissociation constants k a or k ai of indicators. Find examples of titration experiments and tips to improve your results. Demonstrate how to select the proper indicator for a titration experiment; The two most common indicators that are. Indicator In A Titration.
From mungfali.com
Acid Base Titration Indicator Indicator In A Titration Determine the acidic dissociation constants k a or k ai of indicators. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. This page assumes that you know about ph curves for all the. Both indicators change colour over. Indicator In A Titration.
From www.slideshare.net
Acid base titration Indicator In A Titration Find examples of titration experiments and tips to improve your results. This page assumes that you know about ph curves for all the. Demonstrate how to select the proper indicator for a titration experiment; The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Determine the acidic dissociation constants k a or k ai of. Indicator In A Titration.
From www.youtube.com
DOUBLE INDICATOR TITRATION PRACTICAL 2 YouTube Indicator In A Titration The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. Find examples of titration experiments and tips to improve your results. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. An. Indicator In A Titration.
From www.slideserve.com
PPT Indicators for AcidBase Titrations (Sec. 96) PowerPoint Indicator In A Titration Demonstrate how to select the proper indicator for a titration experiment; In the strong acid titration,. This page assumes that you know about ph curves for all the. Determine the acidic dissociation constants k a or k ai of indicators. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Learn what titration is, how. Indicator In A Titration.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Atoms First Indicator In A Titration Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. An indicator is a chemical that changes color when a titration reaches its endpoint. Find examples of titration experiments and tips to improve your results. Both indicators change colour over a specific ph range. The two most common indicators that are used in titrations. Indicator In A Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Indicator In A Titration The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. An indicator is a chemical that changes color when a titration reaches its endpoint. Demonstrate how to select the proper indicator for a titration experiment; Determine the. Indicator In A Titration.
From www.youtube.com
HOW TO DO DOUBLE INDICATOR TITRATION EXPERIMENTS.Chemistry YouTube Indicator In A Titration The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. In the strong acid titration,. This page assumes that you know about ph curves for all the. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Find examples of titration experiments and tips to improve your results.. Indicator In A Titration.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Indicator In A Titration This page assumes that you know about ph curves for all the. Demonstrate how to select the proper indicator for a titration experiment; In the strong acid titration,. Determine the acidic dissociation constants k a or k ai of indicators. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. An indicator is. Indicator In A Titration.
From theedge.com.hk
Chemistry How To Titration The Edge Indicator In A Titration The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Find examples of titration experiments and tips to improve your results. Both indicators change colour over a specific ph range. Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. Determine the acidic dissociation constants k a or k. Indicator In A Titration.
From mungfali.com
Titration Indicators Indicator In A Titration This page assumes that you know about ph curves for all the. Determine the acidic dissociation constants k a or k ai of indicators. Demonstrate how to select the proper indicator for a titration experiment; The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The titration curves shown in figure 14.20 illustrate the choice. Indicator In A Titration.
From www.youtube.com
MJC Chemistry Titration Using a Bromothymol Blue Indicator YouTube Indicator In A Titration Determine the acidic dissociation constants k a or k ai of indicators. Demonstrate how to select the proper indicator for a titration experiment; This page assumes that you know about ph curves for all the. Both indicators change colour over a specific ph range. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The. Indicator In A Titration.
From www.studocu.com
Experiment 3 lectures DoubleIndicator Titration Method ACIDBASE Indicator In A Titration Find examples of titration experiments and tips to improve your results. Determine the acidic dissociation constants k a or k ai of indicators. An indicator is a chemical that changes color when a titration reaches its endpoint. In the strong acid titration,. This page assumes that you know about ph curves for all the. The two most common indicators that. Indicator In A Titration.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Indicator In A Titration Find examples of titration experiments and tips to improve your results. An indicator is a chemical that changes color when a titration reaches its endpoint. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Both indicators change colour over a specific ph range. Determine the acidic dissociation constants k a or k ai of. Indicator In A Titration.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Indicator In A Titration In the strong acid titration,. This page assumes that you know about ph curves for all the. An indicator is a chemical that changes color when a titration reaches its endpoint. Demonstrate how to select the proper indicator for a titration experiment; The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. Both. Indicator In A Titration.
From glossary.periodni.com
Indicator Chemistry Dictionary & Glossary Indicator In A Titration In the strong acid titration,. This page assumes that you know about ph curves for all the. Find examples of titration experiments and tips to improve your results. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. An indicator is a chemical that changes color when a titration reaches its endpoint. The. Indicator In A Titration.
From www.reagent.co.uk
How is Titration Used in the Pharmaceutical Industry? Indicator In A Titration The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. In the strong acid titration,. Demonstrate how to select the proper indicator for a titration experiment; The two most common indicators that are used in titrations are methyl orange and phenolphthalein. An indicator is a chemical that changes color when a titration reaches. Indicator In A Titration.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID9234620 Indicator In A Titration Both indicators change colour over a specific ph range. Determine the acidic dissociation constants k a or k ai of indicators. Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. This page assumes that you know about ph. Indicator In A Titration.
From www.thesciencehive.co.uk
Acids, Alkalis and Titrations (GCSE) — the science hive Indicator In A Titration Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. Demonstrate how to select the proper indicator for a titration experiment; An indicator is a chemical that changes color when a titration reaches its endpoint. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The two. Indicator In A Titration.
From wisc.pb.unizin.org
D30.1 AcidBase Indicators Chemistry 109 Fall 2021 Indicator In A Titration Find examples of titration experiments and tips to improve your results. Both indicators change colour over a specific ph range. This page assumes that you know about ph curves for all the. In the strong acid titration,. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. Demonstrate how to select the proper. Indicator In A Titration.
From saylordotorg.github.io
AcidBase Titrations Indicator In A Titration The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. Demonstrate how to select the proper indicator for a titration experiment; Determine the acidic dissociation constants k a or k ai of indicators. This page assumes that you know about ph curves for all the. Learn what titration is, how it works, and. Indicator In A Titration.
From www.science-revision.co.uk
Titrations Indicator In A Titration In the strong acid titration,. Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. Demonstrate how to select the proper indicator for a titration experiment; An indicator is a chemical that changes color when a titration reaches its endpoint. This page assumes that you know about ph curves for all the. The titration. Indicator In A Titration.
From mungfali.com
Acid Base Titration Indicator Indicator In A Titration An indicator is a chemical that changes color when a titration reaches its endpoint. This page assumes that you know about ph curves for all the. In the strong acid titration,. Both indicators change colour over a specific ph range. Determine the acidic dissociation constants k a or k ai of indicators. Learn what titration is, how it works, and. Indicator In A Titration.
From chrominfo.blogspot.com
Chrominfo Indicators of complexometric titration Indicator In A Titration Both indicators change colour over a specific ph range. Determine the acidic dissociation constants k a or k ai of indicators. Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. This page assumes that you know about ph curves for all the. An indicator is a chemical that changes color when a titration. Indicator In A Titration.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID2134619 Indicator In A Titration In the strong acid titration,. Determine the acidic dissociation constants k a or k ai of indicators. Demonstrate how to select the proper indicator for a titration experiment; The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Both indicators change colour over a specific ph range. Learn what titration is, how it works, and. Indicator In A Titration.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Indicator In A Titration Demonstrate how to select the proper indicator for a titration experiment; Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. An indicator is a chemical that changes color when a titration reaches its endpoint. Find examples of titration experiments and tips to improve your results. In the strong acid titration,. Determine the acidic. Indicator In A Titration.
From freesvg.org
Redox Titration Using Indicator Free SVG Indicator In A Titration Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. Demonstrate how to select the proper indicator for a titration experiment; In the strong acid titration,. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. An indicator is a chemical that changes color when a titration. Indicator In A Titration.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Indicator In A Titration In the strong acid titration,. Demonstrate how to select the proper indicator for a titration experiment; Find examples of titration experiments and tips to improve your results. Both indicators change colour over a specific ph range. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. An indicator is a chemical that changes. Indicator In A Titration.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Indicator In A Titration Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. This page assumes that you know about ph curves for all the. Demonstrate how to select the proper indicator for a titration experiment; The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. Find examples of titration. Indicator In A Titration.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Indicator In A Titration Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. Demonstrate how to select the proper indicator for a titration experiment; The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. In the strong acid titration,. An indicator is a chemical that changes color when a titration. Indicator In A Titration.
From courses.lumenlearning.com
AcidBase Titrations Boundless Chemistry Indicator In A Titration Determine the acidic dissociation constants k a or k ai of indicators. In the strong acid titration,. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. This page assumes that you know about ph curves for all the.. Indicator In A Titration.
From www.vrogue.co
Titration Indicator Types Procedure Indicators vrogue.co Indicator In A Titration This page assumes that you know about ph curves for all the. An indicator is a chemical that changes color when a titration reaches its endpoint. Find examples of titration experiments and tips to improve your results. In the strong acid titration,. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. Learn. Indicator In A Titration.