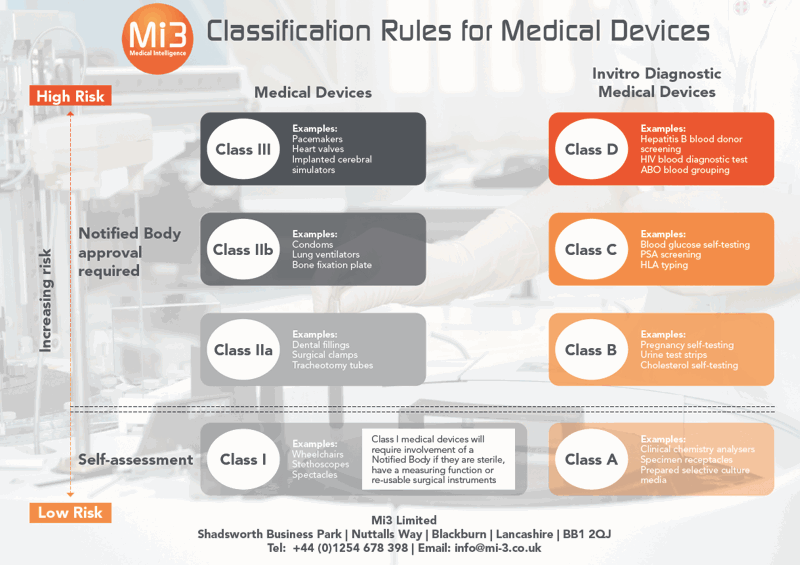
Mhra Medical Devices Classification . If your product is a general medical device or an active implantable device and it is in class iia, iib, iii (or is a class i device that is sterile or has a. The eu has extended the validity of certain directive 93/42/eec on medical devices (eu mdd) and directive 90/385/eec on active implantable. Registration of medical devices with the mhra (the uk competent authority) does not represent any. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. 5.4 if a dispute arises between a manufacturer and an approved body over the classification of a medical device, the mhra. Changing the classification of several types of devices, specifically, increasing the class of certain software as a medical device and aligning ivd classifications with those of. Classification is based on risk, as set out in annex viii of the mdr and annex vii of the ivdr.
from mavink.com
If your product is a general medical device or an active implantable device and it is in class iia, iib, iii (or is a class i device that is sterile or has a. The eu has extended the validity of certain directive 93/42/eec on medical devices (eu mdd) and directive 90/385/eec on active implantable. 5.4 if a dispute arises between a manufacturer and an approved body over the classification of a medical device, the mhra. Classification is based on risk, as set out in annex viii of the mdr and annex vii of the ivdr. Changing the classification of several types of devices, specifically, increasing the class of certain software as a medical device and aligning ivd classifications with those of. Registration of medical devices with the mhra (the uk competent authority) does not represent any. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical.
Mdr Classification Chart
Mhra Medical Devices Classification The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. The eu has extended the validity of certain directive 93/42/eec on medical devices (eu mdd) and directive 90/385/eec on active implantable. Classification is based on risk, as set out in annex viii of the mdr and annex vii of the ivdr. Changing the classification of several types of devices, specifically, increasing the class of certain software as a medical device and aligning ivd classifications with those of. Registration of medical devices with the mhra (the uk competent authority) does not represent any. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. If your product is a general medical device or an active implantable device and it is in class iia, iib, iii (or is a class i device that is sterile or has a. 5.4 if a dispute arises between a manufacturer and an approved body over the classification of a medical device, the mhra.
From gbu-taganskij.ru
MHRA's Guide To The New EU Medical Devices Regulations, 43 OFF Mhra Medical Devices Classification Registration of medical devices with the mhra (the uk competent authority) does not represent any. If your product is a general medical device or an active implantable device and it is in class iia, iib, iii (or is a class i device that is sterile or has a. The eu has extended the validity of certain directive 93/42/eec on medical. Mhra Medical Devices Classification.
From operonstrategist.com
A Comprehensive Guide to MHRA Medical Device Registration (Steps Mhra Medical Devices Classification The eu has extended the validity of certain directive 93/42/eec on medical devices (eu mdd) and directive 90/385/eec on active implantable. Classification is based on risk, as set out in annex viii of the mdr and annex vii of the ivdr. Registration of medical devices with the mhra (the uk competent authority) does not represent any. 5.4 if a dispute. Mhra Medical Devices Classification.
From international.cliniexperts.com
How to register medical devices and IVDs in the UK CliniExperts Mhra Medical Devices Classification If your product is a general medical device or an active implantable device and it is in class iia, iib, iii (or is a class i device that is sterile or has a. Changing the classification of several types of devices, specifically, increasing the class of certain software as a medical device and aligning ivd classifications with those of. Registration. Mhra Medical Devices Classification.
From mungfali.com
Classification Of Medical Devices Mhra Medical Devices Classification The eu has extended the validity of certain directive 93/42/eec on medical devices (eu mdd) and directive 90/385/eec on active implantable. Changing the classification of several types of devices, specifically, increasing the class of certain software as a medical device and aligning ivd classifications with those of. 5.4 if a dispute arises between a manufacturer and an approved body over. Mhra Medical Devices Classification.
From mavink.com
Fda Medical Device Classification Chart Mhra Medical Devices Classification Registration of medical devices with the mhra (the uk competent authority) does not represent any. 5.4 if a dispute arises between a manufacturer and an approved body over the classification of a medical device, the mhra. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. Classification is based on risk, as set out in. Mhra Medical Devices Classification.
From www.scribd.com
MHRA_Guidance List Final Medical Treatments Pharmaceutical Industry Mhra Medical Devices Classification Classification is based on risk, as set out in annex viii of the mdr and annex vii of the ivdr. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. Registration of medical devices with the mhra (the uk competent authority) does not represent any. Changing the classification of several types of devices, specifically, increasing. Mhra Medical Devices Classification.
From www.slideserve.com
PPT MHRA Guidelines. Understanding how to improve practice/safety Mhra Medical Devices Classification 5.4 if a dispute arises between a manufacturer and an approved body over the classification of a medical device, the mhra. Classification is based on risk, as set out in annex viii of the mdr and annex vii of the ivdr. The eu has extended the validity of certain directive 93/42/eec on medical devices (eu mdd) and directive 90/385/eec on. Mhra Medical Devices Classification.
From www.regdesk.co
MHRA Guidance on Registration of Medical Devices RegDesk Mhra Medical Devices Classification Changing the classification of several types of devices, specifically, increasing the class of certain software as a medical device and aligning ivd classifications with those of. 5.4 if a dispute arises between a manufacturer and an approved body over the classification of a medical device, the mhra. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the. Mhra Medical Devices Classification.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Mhra Medical Devices Classification Changing the classification of several types of devices, specifically, increasing the class of certain software as a medical device and aligning ivd classifications with those of. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. 5.4 if a dispute arises between a manufacturer and an approved body over the classification of a medical device,. Mhra Medical Devices Classification.
From www.orielstat.com
Class 1 Medical Device Requirements Oriel STAT A MATRIX Mhra Medical Devices Classification Classification is based on risk, as set out in annex viii of the mdr and annex vii of the ivdr. The eu has extended the validity of certain directive 93/42/eec on medical devices (eu mdd) and directive 90/385/eec on active implantable. Registration of medical devices with the mhra (the uk competent authority) does not represent any. If your product is. Mhra Medical Devices Classification.
From mungfali.com
Classification Of Medical Devices Mhra Medical Devices Classification The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. Registration of medical devices with the mhra (the uk competent authority) does not represent any. Classification is based on risk, as set out in annex viii of the mdr and annex vii of the ivdr. If your product is a general medical device or an. Mhra Medical Devices Classification.
From smartdataweek.com
Medical Device Classification (FDA & EU MDR) SimplerQMS (2024) Mhra Medical Devices Classification If your product is a general medical device or an active implantable device and it is in class iia, iib, iii (or is a class i device that is sterile or has a. Registration of medical devices with the mhra (the uk competent authority) does not represent any. 5.4 if a dispute arises between a manufacturer and an approved body. Mhra Medical Devices Classification.
From www.researchgate.net
MHRA classification of general medical devices Download Table Mhra Medical Devices Classification The eu has extended the validity of certain directive 93/42/eec on medical devices (eu mdd) and directive 90/385/eec on active implantable. Changing the classification of several types of devices, specifically, increasing the class of certain software as a medical device and aligning ivd classifications with those of. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the. Mhra Medical Devices Classification.
From www.youtube.com
StepbyStep Guide How to Get UK MHRA Registration for Medical Devices Mhra Medical Devices Classification Registration of medical devices with the mhra (the uk competent authority) does not represent any. If your product is a general medical device or an active implantable device and it is in class iia, iib, iii (or is a class i device that is sterile or has a. 5.4 if a dispute arises between a manufacturer and an approved body. Mhra Medical Devices Classification.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Mhra Medical Devices Classification The eu has extended the validity of certain directive 93/42/eec on medical devices (eu mdd) and directive 90/385/eec on active implantable. Classification is based on risk, as set out in annex viii of the mdr and annex vii of the ivdr. Changing the classification of several types of devices, specifically, increasing the class of certain software as a medical device. Mhra Medical Devices Classification.
From www.regdesk.co
MHRA Guidance on Medical Software and Applications RegDesk Mhra Medical Devices Classification 5.4 if a dispute arises between a manufacturer and an approved body over the classification of a medical device, the mhra. The eu has extended the validity of certain directive 93/42/eec on medical devices (eu mdd) and directive 90/385/eec on active implantable. Registration of medical devices with the mhra (the uk competent authority) does not represent any. Classification is based. Mhra Medical Devices Classification.
From mavink.com
Mdr Classification Chart Mhra Medical Devices Classification The eu has extended the validity of certain directive 93/42/eec on medical devices (eu mdd) and directive 90/385/eec on active implantable. 5.4 if a dispute arises between a manufacturer and an approved body over the classification of a medical device, the mhra. If your product is a general medical device or an active implantable device and it is in class. Mhra Medical Devices Classification.
From www.cognidox.com
New IVD regulation is coming. are you ready? Mhra Medical Devices Classification The eu has extended the validity of certain directive 93/42/eec on medical devices (eu mdd) and directive 90/385/eec on active implantable. If your product is a general medical device or an active implantable device and it is in class iia, iib, iii (or is a class i device that is sterile or has a. Changing the classification of several types. Mhra Medical Devices Classification.
From www.gov.uk
MHRA reclassifies Viagra Connect tablets to a Pharmacy medicine GOV.UK Mhra Medical Devices Classification 5.4 if a dispute arises between a manufacturer and an approved body over the classification of a medical device, the mhra. Registration of medical devices with the mhra (the uk competent authority) does not represent any. Classification is based on risk, as set out in annex viii of the mdr and annex vii of the ivdr. The eu has extended. Mhra Medical Devices Classification.
From www.lexology.com
The MHRA's recent updates to the regulation of medical devices Lexology Mhra Medical Devices Classification If your product is a general medical device or an active implantable device and it is in class iia, iib, iii (or is a class i device that is sterile or has a. Classification is based on risk, as set out in annex viii of the mdr and annex vii of the ivdr. Changing the classification of several types of. Mhra Medical Devices Classification.
From mavink.com
Medical Device Classification Flowchart Mhra Medical Devices Classification Registration of medical devices with the mhra (the uk competent authority) does not represent any. The eu has extended the validity of certain directive 93/42/eec on medical devices (eu mdd) and directive 90/385/eec on active implantable. Changing the classification of several types of devices, specifically, increasing the class of certain software as a medical device and aligning ivd classifications with. Mhra Medical Devices Classification.
From mavink.com
Mdr Classification Chart Mhra Medical Devices Classification The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. 5.4 if a dispute arises between a manufacturer and an approved body over the classification of a medical device, the mhra. If your product is a general medical device or an active implantable device and it is in class iia, iib, iii (or is a. Mhra Medical Devices Classification.
From www.gov.uk
[Withdrawn] [Withdrawn] Factsheet medical devices overview GOV.UK Mhra Medical Devices Classification The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. Classification is based on risk, as set out in annex viii of the mdr and annex vii of the ivdr. Registration of medical devices with the mhra (the uk competent authority) does not represent any. The eu has extended the validity of certain directive 93/42/eec. Mhra Medical Devices Classification.
From www.linkedin.com
MHRA publish an update on future medical device regulations Mhra Medical Devices Classification Changing the classification of several types of devices, specifically, increasing the class of certain software as a medical device and aligning ivd classifications with those of. 5.4 if a dispute arises between a manufacturer and an approved body over the classification of a medical device, the mhra. The eu has extended the validity of certain directive 93/42/eec on medical devices. Mhra Medical Devices Classification.
From www.qualio.com
The 3 FDA medical device classes differences and examples explained Mhra Medical Devices Classification The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. Classification is based on risk, as set out in annex viii of the mdr and annex vii of the ivdr. Registration of medical devices with the mhra (the uk competent authority) does not represent any. The eu has extended the validity of certain directive 93/42/eec. Mhra Medical Devices Classification.
From coastbiomed.com
UNDERSTANDING MEDICAL EQUIPMENT CLASSIFICATION Coast Biomedical Equipment Mhra Medical Devices Classification The eu has extended the validity of certain directive 93/42/eec on medical devices (eu mdd) and directive 90/385/eec on active implantable. Registration of medical devices with the mhra (the uk competent authority) does not represent any. Changing the classification of several types of devices, specifically, increasing the class of certain software as a medical device and aligning ivd classifications with. Mhra Medical Devices Classification.
From vem-medical.com
Guide to Medical Device Classification Mhra Medical Devices Classification Changing the classification of several types of devices, specifically, increasing the class of certain software as a medical device and aligning ivd classifications with those of. The eu has extended the validity of certain directive 93/42/eec on medical devices (eu mdd) and directive 90/385/eec on active implantable. Classification is based on risk, as set out in annex viii of the. Mhra Medical Devices Classification.
From operonstrategist.com
A Comprehensive Guide to MHRA Medical Device Registration (Steps Mhra Medical Devices Classification If your product is a general medical device or an active implantable device and it is in class iia, iib, iii (or is a class i device that is sterile or has a. The eu has extended the validity of certain directive 93/42/eec on medical devices (eu mdd) and directive 90/385/eec on active implantable. The medicines and healthcare products regulatory. Mhra Medical Devices Classification.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Mhra Medical Devices Classification The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. The eu has extended the validity of certain directive 93/42/eec on medical devices (eu mdd) and directive 90/385/eec on active implantable. Registration of medical devices with the mhra (the uk competent authority) does not represent any. If your product is a general medical device or. Mhra Medical Devices Classification.
From gbu-taganskij.ru
Medical Device Classification According To The MDR Complete, 60 OFF Mhra Medical Devices Classification 5.4 if a dispute arises between a manufacturer and an approved body over the classification of a medical device, the mhra. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. The eu has extended the validity of certain directive 93/42/eec on medical devices (eu mdd) and directive 90/385/eec on active implantable. Changing the classification. Mhra Medical Devices Classification.
From gbu-taganskij.ru
Medical Device Classification According To The MDR Complete, 60 OFF Mhra Medical Devices Classification The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. Changing the classification of several types of devices, specifically, increasing the class of certain software as a medical device and aligning ivd classifications with those of. The eu has extended the validity of certain directive 93/42/eec on medical devices (eu mdd) and directive 90/385/eec on. Mhra Medical Devices Classification.
From casusconsulting.com
UK MHRA 20242025 Medical Device Regulation Plan Casus Consulting Mhra Medical Devices Classification The eu has extended the validity of certain directive 93/42/eec on medical devices (eu mdd) and directive 90/385/eec on active implantable. Registration of medical devices with the mhra (the uk competent authority) does not represent any. If your product is a general medical device or an active implantable device and it is in class iia, iib, iii (or is a. Mhra Medical Devices Classification.
From www.arenasolutions.com
How to Classify Your Medical Device Under the EU MDR and IVDR Arena Mhra Medical Devices Classification The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. Classification is based on risk, as set out in annex viii of the mdr and annex vii of the ivdr. Changing the classification of several types of devices, specifically, increasing the class of certain software as a medical device and aligning ivd classifications with those. Mhra Medical Devices Classification.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Mhra Medical Devices Classification Classification is based on risk, as set out in annex viii of the mdr and annex vii of the ivdr. If your product is a general medical device or an active implantable device and it is in class iia, iib, iii (or is a class i device that is sterile or has a. 5.4 if a dispute arises between a. Mhra Medical Devices Classification.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Mhra Medical Devices Classification 5.4 if a dispute arises between a manufacturer and an approved body over the classification of a medical device, the mhra. Classification is based on risk, as set out in annex viii of the mdr and annex vii of the ivdr. Changing the classification of several types of devices, specifically, increasing the class of certain software as a medical device. Mhra Medical Devices Classification.