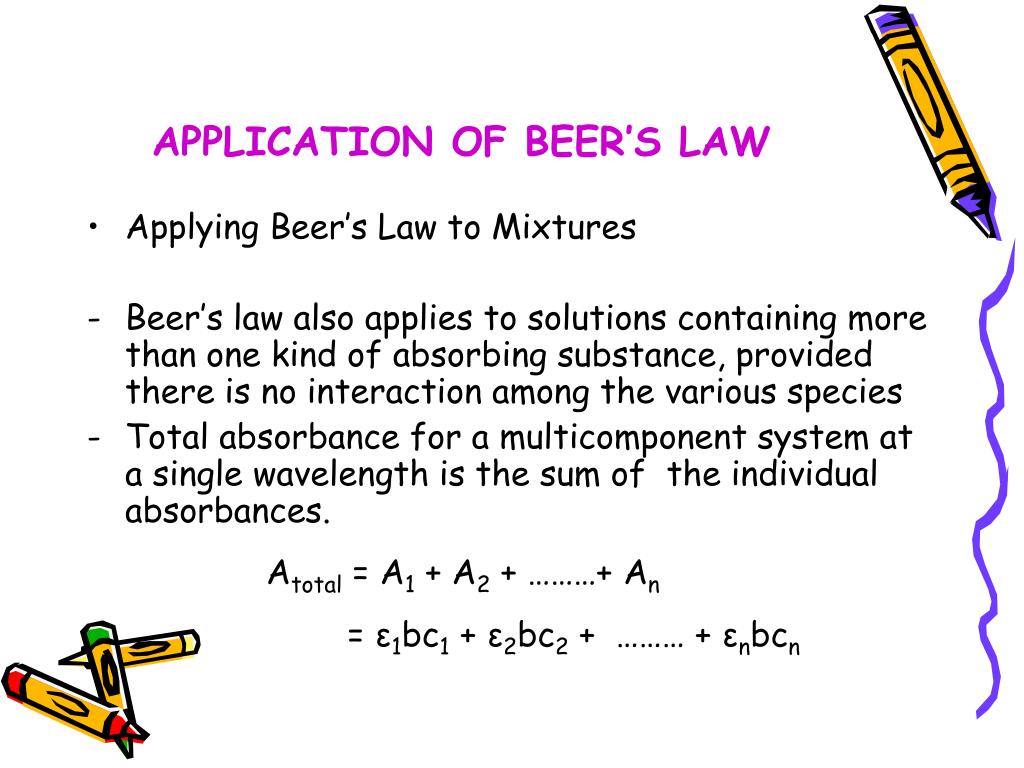
Beer's Law Application To Mixture Analysis . Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). In a 2 component mixture aλ1 =ε1,λ1. A class discussion to improve student comprehension of beer’s law and its application is described. To construct a beer’s law plot we prepare a series of standard solutions—each of which contains a known total concentration of ha—and then measure each solution’s absorbance at the same wavelength. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. A =εbc =−logt =−log i i0 = log i0 i absorbance is additive atotal = a1 + a2. If this light can be. Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in. A demonstration and simple questions that model.
from www.slideserve.com
A class discussion to improve student comprehension of beer’s law and its application is described. Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in. To construct a beer’s law plot we prepare a series of standard solutions—each of which contains a known total concentration of ha—and then measure each solution’s absorbance at the same wavelength. If this light can be. A =εbc =−logt =−log i i0 = log i0 i absorbance is additive atotal = a1 + a2. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. A demonstration and simple questions that model. In a 2 component mixture aλ1 =ε1,λ1. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\).
PPT Introduction to Spectroscopic Methods of Analysis (part 2
Beer's Law Application To Mixture Analysis A =εbc =−logt =−log i i0 = log i0 i absorbance is additive atotal = a1 + a2. A class discussion to improve student comprehension of beer’s law and its application is described. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in. If this light can be. To construct a beer’s law plot we prepare a series of standard solutions—each of which contains a known total concentration of ha—and then measure each solution’s absorbance at the same wavelength. A =εbc =−logt =−log i i0 = log i0 i absorbance is additive atotal = a1 + a2. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In a 2 component mixture aλ1 =ε1,λ1. A demonstration and simple questions that model.
From www.youtube.com
Beer's law Excel Plot Analysis of Unknown Solution Concentrations YouTube Beer's Law Application To Mixture Analysis A =εbc =−logt =−log i i0 = log i0 i absorbance is additive atotal = a1 + a2. A demonstration and simple questions that model. Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in. In a 2 component mixture aλ1 =ε1,λ1. A class discussion to improve student comprehension of beer’s law and its. Beer's Law Application To Mixture Analysis.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Application To Mixture Analysis Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in. A =εbc =−logt =−log i i0 = log i0 i absorbance is additive atotal = a1 + a2. To construct a beer’s law plot we prepare a series of standard solutions—each of which contains a known total concentration of ha—and then measure each solution’s. Beer's Law Application To Mixture Analysis.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download Beer's Law Application To Mixture Analysis To construct a beer’s law plot we prepare a series of standard solutions—each of which contains a known total concentration of ha—and then measure each solution’s absorbance at the same wavelength. Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in. The amount of light that a species absorbs in a spectroscopic transition can. Beer's Law Application To Mixture Analysis.
From www.researchgate.net
Schematics demonstrating the original BeerLambert Law and Modified Beer's Law Application To Mixture Analysis A demonstration and simple questions that model. Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in. To construct a beer’s law plot we prepare a series of standard solutions—each of which contains a known total concentration of ha—and then measure each solution’s absorbance at the same wavelength. A =εbc =−logt =−log i i0. Beer's Law Application To Mixture Analysis.
From www.youtube.com
General Application of Beerlambert law YouTube Beer's Law Application To Mixture Analysis A class discussion to improve student comprehension of beer’s law and its application is described. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). In a 2 component mixture aλ1 =ε1,λ1. To construct a beer’s law plot we prepare a series of standard solutions—each of which contains a known total concentration of ha—and. Beer's Law Application To Mixture Analysis.
From studylib.net
Beer Lambert Law Beer's Law Application To Mixture Analysis To construct a beer’s law plot we prepare a series of standard solutions—each of which contains a known total concentration of ha—and then measure each solution’s absorbance at the same wavelength. If this light can be. In a 2 component mixture aλ1 =ε1,λ1. A class discussion to improve student comprehension of beer’s law and its application is described. Spectrophotometric analysis. Beer's Law Application To Mixture Analysis.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer's Law Application To Mixture Analysis A class discussion to improve student comprehension of beer’s law and its application is described. Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in. To construct a beer’s law plot we prepare a series of standard solutions—each of which contains a known total concentration of ha—and then measure each solution’s absorbance at the. Beer's Law Application To Mixture Analysis.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 Beer's Law Application To Mixture Analysis In a 2 component mixture aλ1 =ε1,λ1. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). To construct a beer’s law plot we prepare a series of standard solutions—each of which contains a known total concentration of ha—and then measure each solution’s absorbance at the same wavelength. If this light can be. A. Beer's Law Application To Mixture Analysis.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples Beer's Law Application To Mixture Analysis A =εbc =−logt =−log i i0 = log i0 i absorbance is additive atotal = a1 + a2. A demonstration and simple questions that model. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is. Beer's Law Application To Mixture Analysis.
From fyooddpjn.blob.core.windows.net
Beer Lambert Law Enzyme Activity at Russell Bingaman blog Beer's Law Application To Mixture Analysis The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. If this light can be. A demonstration and simple questions that model. A =εbc =−logt =−log i i0 = log i0 i absorbance is additive atotal = a1 + a2. A class discussion to improve student comprehension of. Beer's Law Application To Mixture Analysis.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law Application To Mixture Analysis To construct a beer’s law plot we prepare a series of standard solutions—each of which contains a known total concentration of ha—and then measure each solution’s absorbance at the same wavelength. Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in. A class discussion to improve student comprehension of beer’s law and its application. Beer's Law Application To Mixture Analysis.
From joicrwldb.blob.core.windows.net
Beer's Law Biochemistry at Marty Haley blog Beer's Law Application To Mixture Analysis If this light can be. Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in. A class discussion to improve student comprehension of beer’s law and its application is described. A =εbc =−logt =−log i i0 = log i0 i absorbance is additive atotal = a1 + a2. To construct a beer’s law plot. Beer's Law Application To Mixture Analysis.
From www.chegg.com
Solved BeerLambert Law Application Example 1 A compound Beer's Law Application To Mixture Analysis To construct a beer’s law plot we prepare a series of standard solutions—each of which contains a known total concentration of ha—and then measure each solution’s absorbance at the same wavelength. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. A class discussion to improve student comprehension. Beer's Law Application To Mixture Analysis.
From www.youtube.com
Beer Lambert law derivation and usage YouTube Beer's Law Application To Mixture Analysis Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in. A class discussion to improve student comprehension of beer’s law and its application is described. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). In a 2 component mixture aλ1 =ε1,λ1. A =εbc =−logt =−log i i0. Beer's Law Application To Mixture Analysis.
From klaorzemv.blob.core.windows.net
Beer Lambert Law Constant at Rebecca Curtis blog Beer's Law Application To Mixture Analysis The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In a 2 component mixture aλ1 =ε1,λ1. If this light can be. A demonstration and simple questions that model. A class discussion to improve student comprehension of beer’s law and its application is described. Spectrophotometric analysis is essential. Beer's Law Application To Mixture Analysis.
From www.scribd.com
Application of Beer’s Law Quantitative analysis for a single analyte Beer's Law Application To Mixture Analysis Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in. If this light can be. A =εbc =−logt =−log i i0 = log i0 i absorbance is additive atotal = a1 + a2. A demonstration and simple questions that model. In a 2 component mixture aλ1 =ε1,λ1. The amount of light that a species. Beer's Law Application To Mixture Analysis.
From www.youtube.com
Beer's Law Overview YouTube Beer's Law Application To Mixture Analysis In a 2 component mixture aλ1 =ε1,λ1. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). A demonstration and simple questions that model. A class discussion to improve student comprehension of beer’s law and its application is described. A =εbc =−logt =−log i i0 = log i0 i absorbance is additive atotal =. Beer's Law Application To Mixture Analysis.
From chemistnotes.com
Beer lambert law Derivation, deviation, application, and limitations Beer's Law Application To Mixture Analysis A =εbc =−logt =−log i i0 = log i0 i absorbance is additive atotal = a1 + a2. To construct a beer’s law plot we prepare a series of standard solutions—each of which contains a known total concentration of ha—and then measure each solution’s absorbance at the same wavelength. A demonstration and simple questions that model. Spectrophotometric analysis is essential. Beer's Law Application To Mixture Analysis.
From www.slideserve.com
PPT Introduction to Spectroscopic Methods of Analysis (part 2 Beer's Law Application To Mixture Analysis In a 2 component mixture aλ1 =ε1,λ1. If this light can be. Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in. A class discussion to improve student comprehension of beer’s law and its application is described. A =εbc =−logt =−log i i0 = log i0 i absorbance is additive atotal = a1 +. Beer's Law Application To Mixture Analysis.
From chem.libretexts.org
Beer’s Law Chemistry LibreTexts Beer's Law Application To Mixture Analysis If this light can be. A =εbc =−logt =−log i i0 = log i0 i absorbance is additive atotal = a1 + a2. To construct a beer’s law plot we prepare a series of standard solutions—each of which contains a known total concentration of ha—and then measure each solution’s absorbance at the same wavelength. Spectrophotometric analysis is essential for determining. Beer's Law Application To Mixture Analysis.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Application To Mixture Analysis To construct a beer’s law plot we prepare a series of standard solutions—each of which contains a known total concentration of ha—and then measure each solution’s absorbance at the same wavelength. Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in. A class discussion to improve student comprehension of beer’s law and its application. Beer's Law Application To Mixture Analysis.
From www.slideserve.com
PPT Beers Law for a Single Component Sample PowerPoint Presentation Beer's Law Application To Mixture Analysis A =εbc =−logt =−log i i0 = log i0 i absorbance is additive atotal = a1 + a2. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. A class discussion to improve student comprehension of beer’s law and its application is described. To construct a beer’s law. Beer's Law Application To Mixture Analysis.
From joimeihka.blob.core.windows.net
Beer Lambert Law Linear Equation at Kimberly Graves blog Beer's Law Application To Mixture Analysis If this light can be. In a 2 component mixture aλ1 =ε1,λ1. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). A class discussion to improve student comprehension of beer’s law and its application is described. Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in. A. Beer's Law Application To Mixture Analysis.
From www.researchgate.net
Beer's law curves for method A and method B Download Scientific Diagram Beer's Law Application To Mixture Analysis A demonstration and simple questions that model. If this light can be. In a 2 component mixture aλ1 =ε1,λ1. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). A =εbc =−logt. Beer's Law Application To Mixture Analysis.
From www.geeksforgeeks.org
BeerLambert Law Statement, Formula, Equation & Derivation Beer's Law Application To Mixture Analysis A class discussion to improve student comprehension of beer’s law and its application is described. A =εbc =−logt =−log i i0 = log i0 i absorbance is additive atotal = a1 + a2. Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in. The amount of light that a species absorbs in a spectroscopic. Beer's Law Application To Mixture Analysis.
From calculatorghw.blogspot.com
Beer Lambert Law Calculator CALCULATOR GHW Beer's Law Application To Mixture Analysis A =εbc =−logt =−log i i0 = log i0 i absorbance is additive atotal = a1 + a2. A demonstration and simple questions that model. Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in. If this light can be. To construct a beer’s law plot we prepare a series of standard solutions—each of. Beer's Law Application To Mixture Analysis.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer's Law Application To Mixture Analysis To construct a beer’s law plot we prepare a series of standard solutions—each of which contains a known total concentration of ha—and then measure each solution’s absorbance at the same wavelength. A =εbc =−logt =−log i i0 = log i0 i absorbance is additive atotal = a1 + a2. A demonstration and simple questions that model. If this light can. Beer's Law Application To Mixture Analysis.
From www.slideserve.com
PPT UVVIS Molecular Spectroscopy PowerPoint Presentation, free Beer's Law Application To Mixture Analysis The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. A class discussion to improve student comprehension of beer’s law and its application is described. If this light can be. A =εbc =−logt =−log i i0 = log i0 i absorbance is additive atotal = a1 + a2.. Beer's Law Application To Mixture Analysis.
From www.numerade.com
SOLVED T480.1. Beer's law of mixtures The absorbance of a mixture of Beer's Law Application To Mixture Analysis In a 2 component mixture aλ1 =ε1,λ1. To construct a beer’s law plot we prepare a series of standard solutions—each of which contains a known total concentration of ha—and then measure each solution’s absorbance at the same wavelength. A class discussion to improve student comprehension of beer’s law and its application is described. A =εbc =−logt =−log i i0 =. Beer's Law Application To Mixture Analysis.
From www.studocu.com
Cu Analysis Beers Law solution Cu Analysis Part I Beer’s Law In Beer's Law Application To Mixture Analysis In a 2 component mixture aλ1 =ε1,λ1. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. A =εbc =−logt =−log i i0 = log i0 i absorbance is additive atotal = a1 + a2. Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is. Beer's Law Application To Mixture Analysis.
From www.studocu.com
Chem 181 4 Beer's Law Fall 2020 Determining the Concentration of a Beer's Law Application To Mixture Analysis Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). In a 2 component mixture aλ1 =ε1,λ1. A class discussion to improve student comprehension of beer’s law and its application is described. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species.. Beer's Law Application To Mixture Analysis.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law Application To Mixture Analysis Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). A class discussion to improve student comprehension of beer’s law and its application is described. To construct a beer’s law plot we prepare a series of standard solutions—each. Beer's Law Application To Mixture Analysis.
From rrennenextanner.blogspot.com
Beer Lambert Law Ppt rrennenexTanner Beer's Law Application To Mixture Analysis Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. A =εbc =−logt =−log i i0 = log i0 i absorbance is additive atotal = a1 + a2. A class discussion to. Beer's Law Application To Mixture Analysis.
From slideplayer.com
Chapter 13 Chapter 13 Chapter 13 Chapter 13 Chapter 13 Chapter ppt download Beer's Law Application To Mixture Analysis The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in. If this light can be. A demonstration and simple questions that model. Consider monochromatic light of a given intensity incident on a. Beer's Law Application To Mixture Analysis.
From www.chegg.com
Solved A Beer's Law plot is shown here along with the Beer's Law Application To Mixture Analysis A demonstration and simple questions that model. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. A class discussion to improve student comprehension of beer’s law and its application is described. If this light can be. To construct a beer’s law plot we prepare a series of. Beer's Law Application To Mixture Analysis.