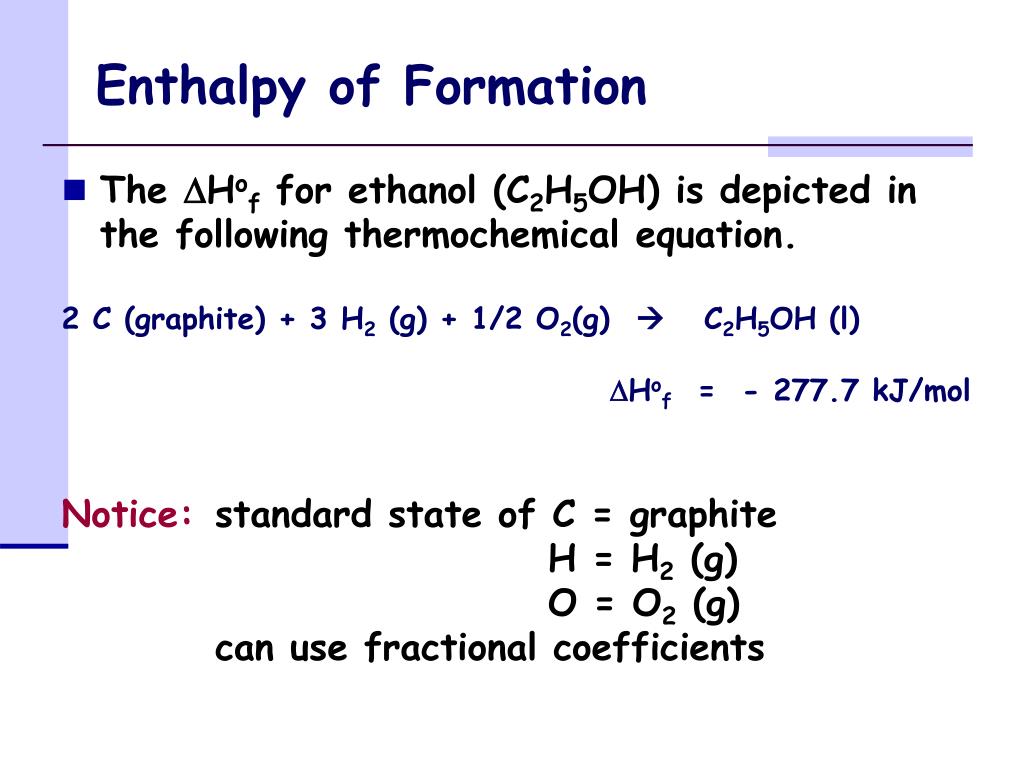
Standard Enthalpy Of Formation Of B2O3 . 54.0 j/(mol k) heat capacity, c p: Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Molar heat capacity at 25 °c: The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c.
from www.slideserve.com
193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Molar heat capacity at 25 °c: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 54.0 j/(mol k) heat capacity, c p: For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation of any element in its standard state is zero by definition.
PPT Enthalpy of Formation PowerPoint Presentation, free download ID
Standard Enthalpy Of Formation Of B2O3 For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 54.0 j/(mol k) heat capacity, c p: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Molar heat capacity at 25 °c:
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation Of B2O3 Molar heat capacity at 25 °c: The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. Standard Enthalpy Of Formation Of B2O3.
From www.numerade.com
(a) Calculate the standard enthalpy of formation of gaseous diborane Standard Enthalpy Of Formation Of B2O3 For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation. Standard Enthalpy Of Formation Of B2O3.
From www.numerade.com
SOLVEDA The standard molar enthalpy of formation of diborane, B2 H6(g Standard Enthalpy Of Formation Of B2O3 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. Molar heat capacity at. Standard Enthalpy Of Formation Of B2O3.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Of B2O3 Molar heat capacity at 25 °c: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm. Standard Enthalpy Of Formation Of B2O3.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation Of B2O3 The standard enthalpy of formation of any element in its standard state is zero by definition. Molar heat capacity at 25 °c: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when. Standard Enthalpy Of Formation Of B2O3.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Of B2O3 For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. 54.0 j/(mol k) heat capacity, c p: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance. Standard Enthalpy Of Formation Of B2O3.
From www.chegg.com
Solved 3.Calculate the standard enthalpy of formation of Standard Enthalpy Of Formation Of B2O3 54.0 j/(mol k) heat capacity, c p: Molar heat capacity at 25 °c: For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. The standard enthalpy of formation is a measure of the energy released or consumed. Standard Enthalpy Of Formation Of B2O3.
From www.researchgate.net
Enthalpies of formation for stable and radical species used in work Standard Enthalpy Of Formation Of B2O3 For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Molar heat capacity. Standard Enthalpy Of Formation Of B2O3.
From www.numerade.com
SOLVED 'The following problems deal with standard enthalpy of Standard Enthalpy Of Formation Of B2O3 The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of any element in its standard state is. Standard Enthalpy Of Formation Of B2O3.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Enthalpy Of Formation Of B2O3 54.0 j/(mol k) heat capacity, c p: The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard. Standard Enthalpy Of Formation Of B2O3.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Of B2O3 54.0 j/(mol k) heat capacity, c p: Molar heat capacity at 25 °c: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of.. Standard Enthalpy Of Formation Of B2O3.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Of B2O3 For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. Molar heat capacity at 25 °c: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is. Standard Enthalpy Of Formation Of B2O3.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Standard Enthalpy Of Formation Of B2O3 The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Molar heat capacity at 25 °c: 193 rows in chemistry and thermodynamics, the standard enthalpy. Standard Enthalpy Of Formation Of B2O3.
From www.slideserve.com
PPT THERMOCHEMISTRY PowerPoint Presentation, free download ID5072129 Standard Enthalpy Of Formation Of B2O3 For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is. Standard Enthalpy Of Formation Of B2O3.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Of B2O3 For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 54.0 j/(mol k) heat capacity, c. Standard Enthalpy Of Formation Of B2O3.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation Of B2O3 The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 54.0 j/(mol k) heat capacity, c p: Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation of any element. Standard Enthalpy Of Formation Of B2O3.
From kaden-chapter.blogspot.com
Standard Enthalpy Of Formation Table Pdf 30+ Pages Summary [1.8mb Standard Enthalpy Of Formation Of B2O3 The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Molar heat capacity at 25 °c: 54.0 j/(mol k) heat capacity, c p: The standard enthalpy of formation is a measure of the. Standard Enthalpy Of Formation Of B2O3.
From www.researchgate.net
Enthalpy of formation, reaction, and of metal oxides and Standard Enthalpy Of Formation Of B2O3 For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in. Standard Enthalpy Of Formation Of B2O3.
From www.slideserve.com
PPT Advanced Thermodynamics Note 3 Heat Effects PowerPoint Standard Enthalpy Of Formation Of B2O3 For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Molar heat capacity at. Standard Enthalpy Of Formation Of B2O3.
From www.chegg.com
Solved Table 1. Standard enthalpies of formation for Standard Enthalpy Of Formation Of B2O3 Molar heat capacity at 25 °c: For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. 54.0 j/(mol k) heat capacity, c p: The standard enthalpy of formation is a measure of the energy released or consumed. Standard Enthalpy Of Formation Of B2O3.
From www.chegg.com
Solved Using the table of standard enthalpies of formation Standard Enthalpy Of Formation Of B2O3 54.0 j/(mol k) heat capacity, c p: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Molar heat capacity at 25 °c: For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is. Standard Enthalpy Of Formation Of B2O3.
From mavink.com
Standard Enthalpy Chart Standard Enthalpy Of Formation Of B2O3 54.0 j/(mol k) heat capacity, c p: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Molar heat capacity at 25 °c: The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone. Standard Enthalpy Of Formation Of B2O3.
From slideplayer.com
Thermochemistry Chemistry that deals with Energy Change ppt download Standard Enthalpy Of Formation Of B2O3 Molar heat capacity at 25 °c: For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is. Standard Enthalpy Of Formation Of B2O3.
From www.chegg.com
Solved Calculate the standard enthalpy of formation of Standard Enthalpy Of Formation Of B2O3 54.0 j/(mol k) heat capacity, c p: Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Molar heat capacity at 25 °c: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard. Standard Enthalpy Of Formation Of B2O3.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Of B2O3 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 54.0 j/(mol k) heat capacity, c p: Molar heat capacity at 25 °c: The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is a measure. Standard Enthalpy Of Formation Of B2O3.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation Of B2O3 54.0 j/(mol k) heat capacity, c p: For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Standard Enthalpy Of Formation Of B2O3.
From worksheetdbblags.z13.web.core.windows.net
How To Find Standard Enthalpy Of Reaction Standard Enthalpy Of Formation Of B2O3 54.0 j/(mol k) heat capacity, c p: For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is. Standard Enthalpy Of Formation Of B2O3.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation Of B2O3 For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in. Standard Enthalpy Of Formation Of B2O3.
From mungfali.com
Enthalpies Of Formation Table Standard Enthalpy Of Formation Of B2O3 54.0 j/(mol k) heat capacity, c p: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy change of formation (data. Standard Enthalpy Of Formation Of B2O3.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Of B2O3 Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of.. Standard Enthalpy Of Formation Of B2O3.
From www.numerade.com
Calculate the standard enthalpy of formation of gaseous diborane (B2H6 Standard Enthalpy Of Formation Of B2O3 Molar heat capacity at 25 °c: Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 54.0 j/(mol k) heat capacity, c p: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy. Standard Enthalpy Of Formation Of B2O3.
From mavink.com
Standard Enthalpy Chart Standard Enthalpy Of Formation Of B2O3 Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of.. Standard Enthalpy Of Formation Of B2O3.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation of gaseous Standard Enthalpy Of Formation Of B2O3 For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows in chemistry and thermodynamics,. Standard Enthalpy Of Formation Of B2O3.
From www.numerade.com
SOLVEDCalculate the standard enthalpy of formation of gaseous diborane Standard Enthalpy Of Formation Of B2O3 Molar heat capacity at 25 °c: Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation of any element in its standard state is zero by definition. 54.0 j/(mol k) heat capacity, c p: The standard enthalpy of formation is a measure of the. Standard Enthalpy Of Formation Of B2O3.
From www.researchgate.net
Molecular weight, standard enthalpy of formation and thermal Standard Enthalpy Of Formation Of B2O3 For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. Molar heat capacity at 25 °c: The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation. Standard Enthalpy Of Formation Of B2O3.