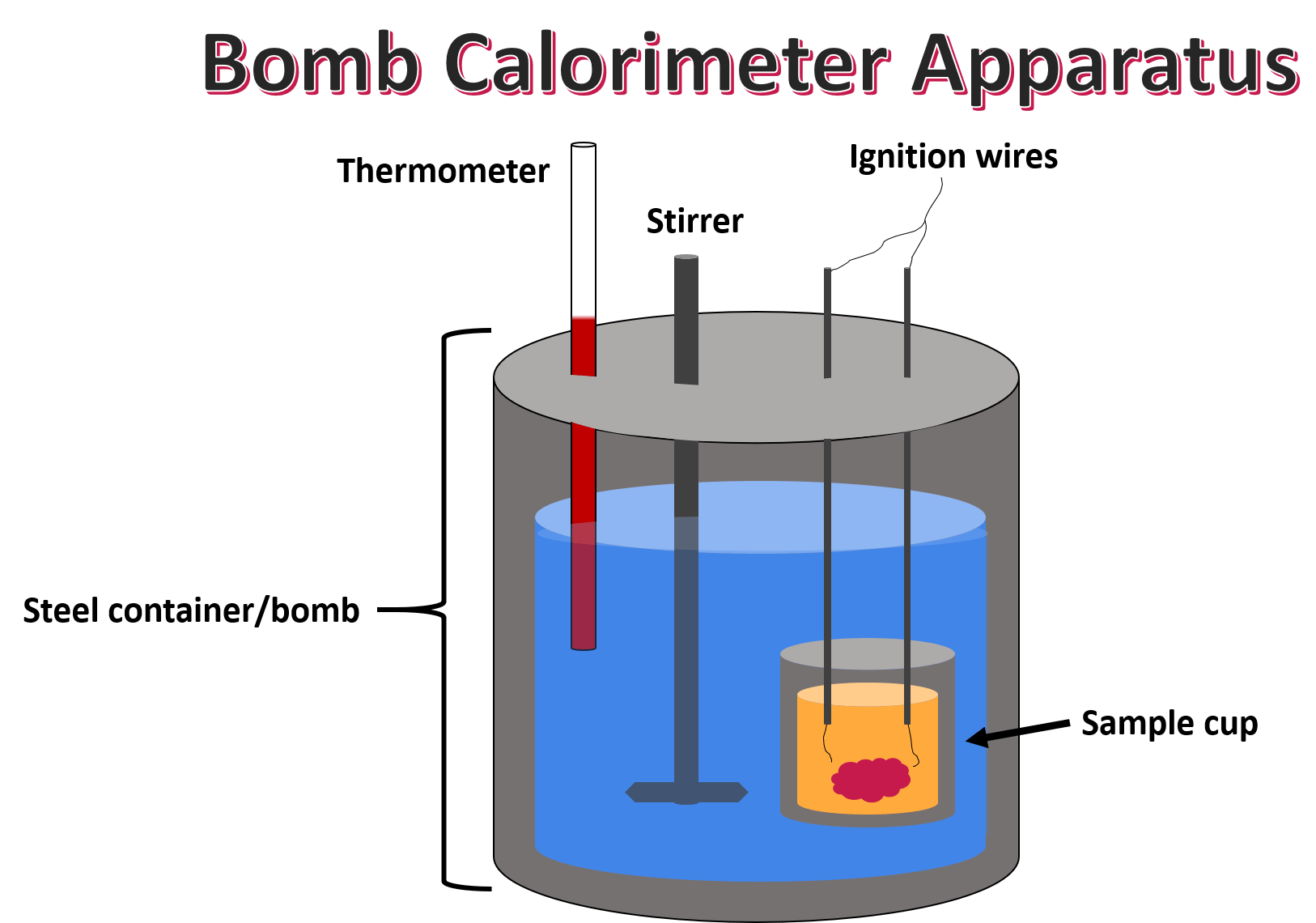
Constant Pressure Calorimeter Experiment . Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). Measuring a reaction enthalpy in a coffee cup calorimeter. To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. In that case, the gaseous pressure above the solution remains constant, and. A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure. _____ _____ (3) you will determine the calorimeter constant ccal. _____ _____ (2) how is heat q related to enthalpy change δh at constant pressure? Since hxn is given by q at constant pressure, and the laboratory is a constant. (1) what is the purpose of this experiment? To apply these \( \delta h\) values in a hess’s law calculation to.
from martinfersbanks.blogspot.com
In that case, the gaseous pressure above the solution remains constant, and. A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure. Since hxn is given by q at constant pressure, and the laboratory is a constant. Measuring a reaction enthalpy in a coffee cup calorimeter. _____ _____ (2) how is heat q related to enthalpy change δh at constant pressure? To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. _____ _____ (3) you will determine the calorimeter constant ccal. To apply these \( \delta h\) values in a hess’s law calculation to. (1) what is the purpose of this experiment? Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q).
Is a Bomb Calorimeter Constant Pressure
Constant Pressure Calorimeter Experiment In that case, the gaseous pressure above the solution remains constant, and. To apply these \( \delta h\) values in a hess’s law calculation to. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). Since hxn is given by q at constant pressure, and the laboratory is a constant. _____ _____ (2) how is heat q related to enthalpy change δh at constant pressure? A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure. (1) what is the purpose of this experiment? In that case, the gaseous pressure above the solution remains constant, and. To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. _____ _____ (3) you will determine the calorimeter constant ccal. Measuring a reaction enthalpy in a coffee cup calorimeter.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or Constant Pressure Calorimeter Experiment Measuring a reaction enthalpy in a coffee cup calorimeter. _____ _____ (2) how is heat q related to enthalpy change δh at constant pressure? _____ _____ (3) you will determine the calorimeter constant ccal. To apply these \( \delta h\) values in a hess’s law calculation to. To experimentally measure the \( \delta h\) values of two reactions using the. Constant Pressure Calorimeter Experiment.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Constant Pressure Calorimeter Experiment To apply these \( \delta h\) values in a hess’s law calculation to. To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. Since hxn is given by q at constant pressure, and the laboratory is a constant. Measuring a reaction enthalpy in a coffee cup calorimeter. _____ _____ (2) how is. Constant Pressure Calorimeter Experiment.
From courses.lumenlearning.com
Calorimetry Chemistry I Constant Pressure Calorimeter Experiment To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. In that case, the gaseous pressure above the solution remains constant, and. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). Measuring a reaction enthalpy in a coffee. Constant Pressure Calorimeter Experiment.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or Constant Pressure Calorimeter Experiment A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure. Since hxn is given by q at constant pressure, and the laboratory is a constant. (1) what is the purpose of this experiment? Measuring a reaction enthalpy in a coffee cup calorimeter. Constant pressure calorimetry is used for reactions carried out. Constant Pressure Calorimeter Experiment.
From martinfersbanks.blogspot.com
Is a Bomb Calorimeter Constant Pressure Constant Pressure Calorimeter Experiment (1) what is the purpose of this experiment? _____ _____ (3) you will determine the calorimeter constant ccal. _____ _____ (2) how is heat q related to enthalpy change δh at constant pressure? To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. A simple coffee cup calorimeter measures change in enthalpy. Constant Pressure Calorimeter Experiment.
From www.researchgate.net
Schematic of constant pressure calorimeter for APW classification Constant Pressure Calorimeter Experiment A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry.. Constant Pressure Calorimeter Experiment.
From www.pearson.com
Chapter 09 16 Constant Pressure Calorimetry (coffee cup) Channels Constant Pressure Calorimeter Experiment Measuring a reaction enthalpy in a coffee cup calorimeter. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). (1) what is the purpose of this experiment? To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. A simple. Constant Pressure Calorimeter Experiment.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Constant Pressure Calorimeter Experiment To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. Measuring a reaction enthalpy in a coffee cup calorimeter. To apply these \( \delta h\) values in a hess’s law calculation to. A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure. In. Constant Pressure Calorimeter Experiment.
From www.studocu.com
ACT 3 Constant Pressure Calorimetry ACTIVITY 3 CONSTANT PRESSURE Constant Pressure Calorimeter Experiment To apply these \( \delta h\) values in a hess’s law calculation to. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). _____ _____ (3) you will determine the calorimeter constant ccal. Since hxn is given by q at constant pressure, and the laboratory is a. Constant Pressure Calorimeter Experiment.
From www.bartleby.com
Answered In the laboratory a "coffee cup"… bartleby Constant Pressure Calorimeter Experiment _____ _____ (2) how is heat q related to enthalpy change δh at constant pressure? _____ _____ (3) you will determine the calorimeter constant ccal. A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure. Measuring a reaction enthalpy in a coffee cup calorimeter. In that case, the gaseous pressure above. Constant Pressure Calorimeter Experiment.
From www.youtube.com
Coffee Cup Calorimeter Calculate Enthalpy Change, Constant Pressure Constant Pressure Calorimeter Experiment In that case, the gaseous pressure above the solution remains constant, and. _____ _____ (2) how is heat q related to enthalpy change δh at constant pressure? To apply these \( \delta h\) values in a hess’s law calculation to. To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. (1) what. Constant Pressure Calorimeter Experiment.
From users.highland.edu
Calorimetry Constant Pressure Calorimeter Experiment Since hxn is given by q at constant pressure, and the laboratory is a constant. To apply these \( \delta h\) values in a hess’s law calculation to. _____ _____ (3) you will determine the calorimeter constant ccal. A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure. Measuring a reaction. Constant Pressure Calorimeter Experiment.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Constant Pressure Calorimeter Experiment (1) what is the purpose of this experiment? Measuring a reaction enthalpy in a coffee cup calorimeter. _____ _____ (3) you will determine the calorimeter constant ccal. _____ _____ (2) how is heat q related to enthalpy change δh at constant pressure? Since hxn is given by q at constant pressure, and the laboratory is a constant. In that case,. Constant Pressure Calorimeter Experiment.
From www.slideserve.com
PPT Zumdahl Chapter 6 PowerPoint Presentation, free download ID4269919 Constant Pressure Calorimeter Experiment To apply these \( \delta h\) values in a hess’s law calculation to. A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure. _____ _____ (3) you will determine the calorimeter constant ccal. To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry.. Constant Pressure Calorimeter Experiment.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Constant Pressure Calorimeter Experiment Measuring a reaction enthalpy in a coffee cup calorimeter. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). _____ _____ (2) how is heat q related to enthalpy change δh at constant pressure? Since hxn is given by q at constant pressure, and the laboratory is. Constant Pressure Calorimeter Experiment.
From app.jove.com
Constant Pressure Calorimeter/ Coffee Cup Calorimeter Concept Constant Pressure Calorimeter Experiment _____ _____ (2) how is heat q related to enthalpy change δh at constant pressure? Measuring a reaction enthalpy in a coffee cup calorimeter. In that case, the gaseous pressure above the solution remains constant, and. Since hxn is given by q at constant pressure, and the laboratory is a constant. To apply these \( \delta h\) values in a. Constant Pressure Calorimeter Experiment.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or Constant Pressure Calorimeter Experiment To apply these \( \delta h\) values in a hess’s law calculation to. To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. In that case, the gaseous pressure above the solution remains constant, and. _____ _____ (2) how is heat q related to enthalpy change δh at constant pressure? _____ _____. Constant Pressure Calorimeter Experiment.
From www.youtube.com
1A 6.7 ConstantPressure Calorimetry YouTube Constant Pressure Calorimeter Experiment Measuring a reaction enthalpy in a coffee cup calorimeter. A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure. To apply these \( \delta h\) values in a hess’s law calculation to. Since hxn is given by q at constant pressure, and the laboratory is a constant. _____ _____ (2) how. Constant Pressure Calorimeter Experiment.
From kaffee.50webs.com
Lab Calorimetry Constant Pressure Calorimeter Experiment A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure. _____ _____ (2) how is heat q related to enthalpy change δh at constant pressure? Since hxn is given by q at constant pressure, and the laboratory is a constant. To experimentally measure the \( \delta h\) values of two reactions. Constant Pressure Calorimeter Experiment.
From www.w3schools.blog
Cp, Cv calorimetry W3schools Constant Pressure Calorimeter Experiment To apply these \( \delta h\) values in a hess’s law calculation to. Since hxn is given by q at constant pressure, and the laboratory is a constant. (1) what is the purpose of this experiment? A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure. In that case, the gaseous. Constant Pressure Calorimeter Experiment.
From saylordotorg.github.io
Calorimetry Constant Pressure Calorimeter Experiment _____ _____ (2) how is heat q related to enthalpy change δh at constant pressure? To apply these \( \delta h\) values in a hess’s law calculation to. In that case, the gaseous pressure above the solution remains constant, and. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to. Constant Pressure Calorimeter Experiment.
From www.pinterest.com
ConstantPressure Calorimetery "coffeecup" calorimeter often used to Constant Pressure Calorimeter Experiment In that case, the gaseous pressure above the solution remains constant, and. _____ _____ (2) how is heat q related to enthalpy change δh at constant pressure? Measuring a reaction enthalpy in a coffee cup calorimeter. To apply these \( \delta h\) values in a hess’s law calculation to. Constant pressure calorimetry is used for reactions carried out under constant. Constant Pressure Calorimeter Experiment.
From www.numerade.com
SOLVED In the laboratory a "coffee cup calorimeter, Or constant Constant Pressure Calorimeter Experiment A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure. In that case, the gaseous pressure above the solution remains constant, and. Since hxn is given by q at constant pressure, and the laboratory is a constant. Measuring a reaction enthalpy in a coffee cup calorimeter. (1) what is the purpose. Constant Pressure Calorimeter Experiment.
From learninglibjohn88.z19.web.core.windows.net
Coffee Cup Calorimetry Experiment Constant Pressure Calorimeter Experiment _____ _____ (3) you will determine the calorimeter constant ccal. To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). Since hxn is given by q at constant pressure,. Constant Pressure Calorimeter Experiment.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Constant Pressure Calorimeter Experiment Measuring a reaction enthalpy in a coffee cup calorimeter. Since hxn is given by q at constant pressure, and the laboratory is a constant. To apply these \( \delta h\) values in a hess’s law calculation to. _____ _____ (3) you will determine the calorimeter constant ccal. In that case, the gaseous pressure above the solution remains constant, and. (1). Constant Pressure Calorimeter Experiment.
From www.studocu.com
Lab02Calorimetry Lectures and activities LABORATORY ACTIVITY 02 Constant Pressure Calorimeter Experiment (1) what is the purpose of this experiment? To apply these \( \delta h\) values in a hess’s law calculation to. A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to. Constant Pressure Calorimeter Experiment.
From martinfersbanks.blogspot.com
Is a Bomb Calorimeter Constant Pressure Constant Pressure Calorimeter Experiment Since hxn is given by q at constant pressure, and the laboratory is a constant. In that case, the gaseous pressure above the solution remains constant, and. (1) what is the purpose of this experiment? _____ _____ (2) how is heat q related to enthalpy change δh at constant pressure? A simple coffee cup calorimeter measures change in enthalpy of. Constant Pressure Calorimeter Experiment.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download Constant Pressure Calorimeter Experiment Since hxn is given by q at constant pressure, and the laboratory is a constant. To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. To apply these \( \delta h\) values in a hess’s law calculation to. A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in. Constant Pressure Calorimeter Experiment.
From maestrovirtuale.com
Calorimetria o que você estuda e aplicações Constant Pressure Calorimeter Experiment In that case, the gaseous pressure above the solution remains constant, and. A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure. Measuring a reaction enthalpy in a coffee cup calorimeter. Since hxn is given by q at constant pressure, and the laboratory is a constant. _____ _____ (3) you will. Constant Pressure Calorimeter Experiment.
From www.youtube.com
AP Chemistry Thermochemistry I Part 4 Constant Pressure Calorimetry Constant Pressure Calorimeter Experiment A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure. Since hxn is given by q at constant pressure, and the laboratory is a constant. (1) what is the purpose of this experiment? To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry.. Constant Pressure Calorimeter Experiment.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Constant Pressure Calorimeter Experiment A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure. To apply these \( \delta h\) values in a hess’s law calculation to. In that case, the gaseous pressure above the solution remains constant, and. _____ _____ (2) how is heat q related to enthalpy change δh at constant pressure? To. Constant Pressure Calorimeter Experiment.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Constant Pressure Calorimeter Experiment In that case, the gaseous pressure above the solution remains constant, and. _____ _____ (3) you will determine the calorimeter constant ccal. (1) what is the purpose of this experiment? _____ _____ (2) how is heat q related to enthalpy change δh at constant pressure? Since hxn is given by q at constant pressure, and the laboratory is a constant.. Constant Pressure Calorimeter Experiment.
From www.slideserve.com
PPT Chapter 12 “Heat in Chemical Reactions” PowerPoint Presentation Constant Pressure Calorimeter Experiment In that case, the gaseous pressure above the solution remains constant, and. _____ _____ (3) you will determine the calorimeter constant ccal. To apply these \( \delta h\) values in a hess’s law calculation to. _____ _____ (2) how is heat q related to enthalpy change δh at constant pressure? Measuring a reaction enthalpy in a coffee cup calorimeter. Since. Constant Pressure Calorimeter Experiment.
From www.numerade.com
SOLVED In the laboratory a "coffee cup" calorimeter, or constant Constant Pressure Calorimeter Experiment _____ _____ (3) you will determine the calorimeter constant ccal. Since hxn is given by q at constant pressure, and the laboratory is a constant. _____ _____ (2) how is heat q related to enthalpy change δh at constant pressure? To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. A simple. Constant Pressure Calorimeter Experiment.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2692866 Constant Pressure Calorimeter Experiment A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure. To experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. In that case, the gaseous pressure above the solution remains constant, and. _____ _____ (2) how is heat q related to enthalpy change. Constant Pressure Calorimeter Experiment.