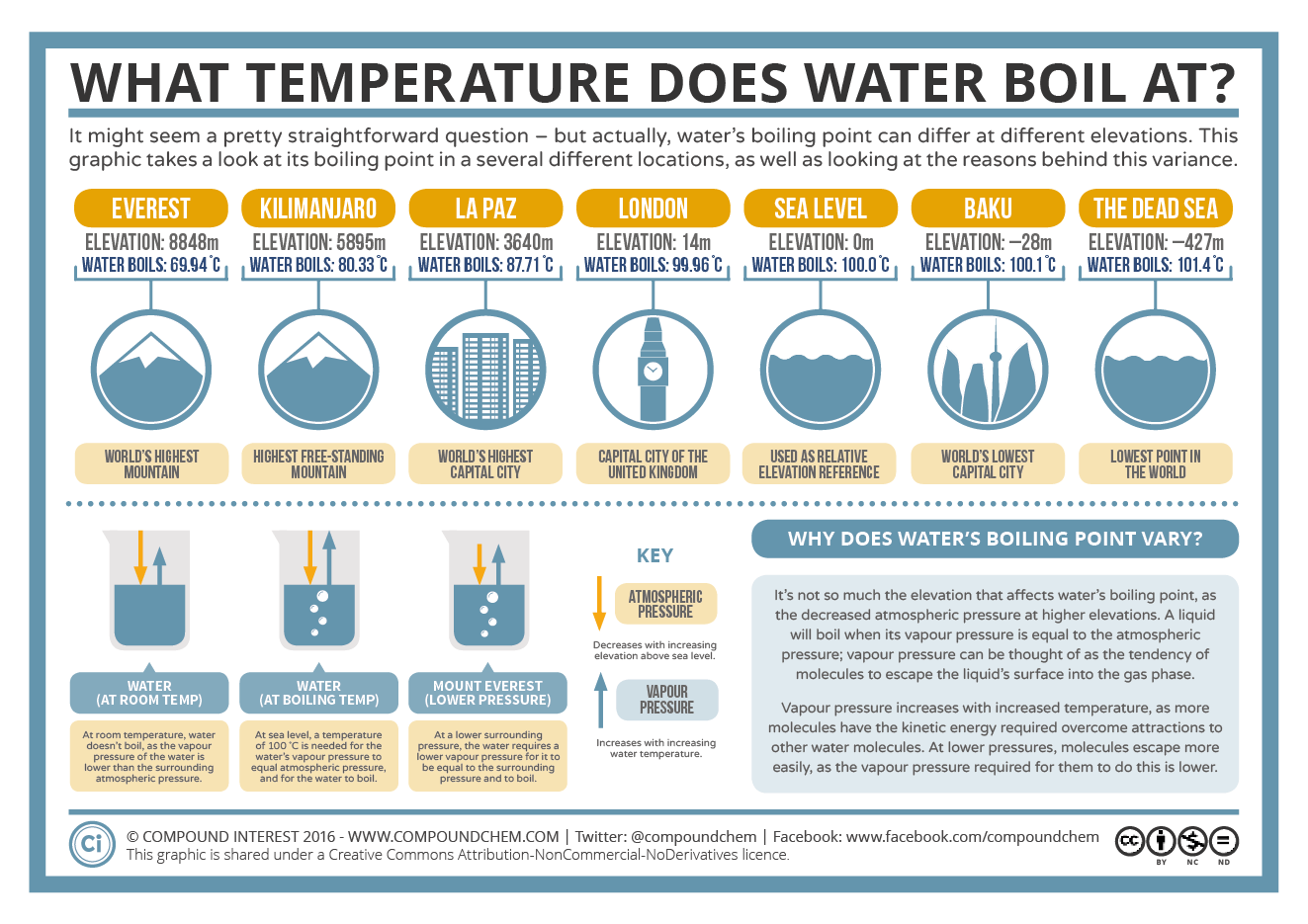
Does Water Boil Hotter At Sea Level . If you were to boil water that was pressurized greater than sea level atmospheric pressure, then yes, it can get hotter than 100 °c. At sea level, vapour pressure is equal to the atmospheric pressure at 100 ˚c, and so this is the temperature at which water boils. At sea level, water boils at 100 °c (212 °f) and freezes at 0 °c (32 °f). At sea level at a pressure of 1.013 bar, water begins to boil at a temperature of 100 °c. As we move higher into the atmosphere and the atmospheric pressure drops, so too does the amount of vapour pressure required for a liquid to boil. Boiling point depends on pressure. When water is heated to the boiling point, it reaches 100°c at sea level, and it transforms from a liquid to a gas, which we. However, on mount everest at an altitude of. Using this water boiling point calculator, boiling point at sea level (1 atm or 29.92 inches of hg) is approximately 212 °f whereas at a height of. The temperature at which water boils isn’t the same everywhere. The answer is the water reaches its boiling point temperature and stays there.
from www.compoundchem.com
Using this water boiling point calculator, boiling point at sea level (1 atm or 29.92 inches of hg) is approximately 212 °f whereas at a height of. If you were to boil water that was pressurized greater than sea level atmospheric pressure, then yes, it can get hotter than 100 °c. As we move higher into the atmosphere and the atmospheric pressure drops, so too does the amount of vapour pressure required for a liquid to boil. At sea level, vapour pressure is equal to the atmospheric pressure at 100 ˚c, and so this is the temperature at which water boils. The answer is the water reaches its boiling point temperature and stays there. However, on mount everest at an altitude of. At sea level at a pressure of 1.013 bar, water begins to boil at a temperature of 100 °c. When water is heated to the boiling point, it reaches 100°c at sea level, and it transforms from a liquid to a gas, which we. The temperature at which water boils isn’t the same everywhere. Boiling point depends on pressure.
What Temperature Does Water Boil At? Boiling Point & Elevation
Does Water Boil Hotter At Sea Level Boiling point depends on pressure. As we move higher into the atmosphere and the atmospheric pressure drops, so too does the amount of vapour pressure required for a liquid to boil. When water is heated to the boiling point, it reaches 100°c at sea level, and it transforms from a liquid to a gas, which we. If you were to boil water that was pressurized greater than sea level atmospheric pressure, then yes, it can get hotter than 100 °c. At sea level, water boils at 100 °c (212 °f) and freezes at 0 °c (32 °f). At sea level at a pressure of 1.013 bar, water begins to boil at a temperature of 100 °c. The temperature at which water boils isn’t the same everywhere. However, on mount everest at an altitude of. The answer is the water reaches its boiling point temperature and stays there. At sea level, vapour pressure is equal to the atmospheric pressure at 100 ˚c, and so this is the temperature at which water boils. Using this water boiling point calculator, boiling point at sea level (1 atm or 29.92 inches of hg) is approximately 212 °f whereas at a height of. Boiling point depends on pressure.
From sciencenotes.org
How to Boil Water at Room Temperature Does Water Boil Hotter At Sea Level At sea level, water boils at 100 °c (212 °f) and freezes at 0 °c (32 °f). At sea level, vapour pressure is equal to the atmospheric pressure at 100 ˚c, and so this is the temperature at which water boils. Using this water boiling point calculator, boiling point at sea level (1 atm or 29.92 inches of hg) is. Does Water Boil Hotter At Sea Level.
From grizly.com
At sea level, what temperature does water boil? QuizGriz Does Water Boil Hotter At Sea Level The answer is the water reaches its boiling point temperature and stays there. At sea level, water boils at 100 °c (212 °f) and freezes at 0 °c (32 °f). When water is heated to the boiling point, it reaches 100°c at sea level, and it transforms from a liquid to a gas, which we. Boiling point depends on pressure.. Does Water Boil Hotter At Sea Level.
From www.lihpao.com
How Hot Does Water Have to Be to Boil? Exploring the Science Behind It Does Water Boil Hotter At Sea Level Using this water boiling point calculator, boiling point at sea level (1 atm or 29.92 inches of hg) is approximately 212 °f whereas at a height of. When water is heated to the boiling point, it reaches 100°c at sea level, and it transforms from a liquid to a gas, which we. At sea level, vapour pressure is equal to. Does Water Boil Hotter At Sea Level.
From aweseas.blogspot.com
Boiling Point At Sea Level Vs Altitude Does Water Boil Hotter At Sea Level At sea level at a pressure of 1.013 bar, water begins to boil at a temperature of 100 °c. However, on mount everest at an altitude of. At sea level, vapour pressure is equal to the atmospheric pressure at 100 ˚c, and so this is the temperature at which water boils. Using this water boiling point calculator, boiling point at. Does Water Boil Hotter At Sea Level.
From fyozkayiz.blob.core.windows.net
Why Does Water Boil At 100 Degrees Celsius At Sea Level at Jesus Newman Does Water Boil Hotter At Sea Level At sea level, water boils at 100 °c (212 °f) and freezes at 0 °c (32 °f). The answer is the water reaches its boiling point temperature and stays there. When water is heated to the boiling point, it reaches 100°c at sea level, and it transforms from a liquid to a gas, which we. At sea level, vapour pressure. Does Water Boil Hotter At Sea Level.
From www.neuronmagazine.com
What Temperature Does Water Boil At 101 Does Water Boil Hotter At Sea Level However, on mount everest at an altitude of. The answer is the water reaches its boiling point temperature and stays there. When water is heated to the boiling point, it reaches 100°c at sea level, and it transforms from a liquid to a gas, which we. As we move higher into the atmosphere and the atmospheric pressure drops, so too. Does Water Boil Hotter At Sea Level.
From eatmorebutter.com
How Does Water Boil at a Higher Temperature Than Butter A Guide Eat Does Water Boil Hotter At Sea Level However, on mount everest at an altitude of. Boiling point depends on pressure. When water is heated to the boiling point, it reaches 100°c at sea level, and it transforms from a liquid to a gas, which we. At sea level, water boils at 100 °c (212 °f) and freezes at 0 °c (32 °f). The answer is the water. Does Water Boil Hotter At Sea Level.
From slideplayer.com
Chapter 3 Review. ppt download Does Water Boil Hotter At Sea Level Using this water boiling point calculator, boiling point at sea level (1 atm or 29.92 inches of hg) is approximately 212 °f whereas at a height of. As we move higher into the atmosphere and the atmospheric pressure drops, so too does the amount of vapour pressure required for a liquid to boil. Boiling point depends on pressure. The answer. Does Water Boil Hotter At Sea Level.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level Does Water Boil Hotter At Sea Level The answer is the water reaches its boiling point temperature and stays there. When water is heated to the boiling point, it reaches 100°c at sea level, and it transforms from a liquid to a gas, which we. If you were to boil water that was pressurized greater than sea level atmospheric pressure, then yes, it can get hotter than. Does Water Boil Hotter At Sea Level.
From www.scienceabc.com
Why Does Water Boil Quickly At High Altitudes? » ScienceABC Does Water Boil Hotter At Sea Level At sea level, water boils at 100 °c (212 °f) and freezes at 0 °c (32 °f). However, on mount everest at an altitude of. Boiling point depends on pressure. At sea level at a pressure of 1.013 bar, water begins to boil at a temperature of 100 °c. When water is heated to the boiling point, it reaches 100°c. Does Water Boil Hotter At Sea Level.
From www.slideserve.com
PPT Ch15 Global Circulation and Weather PowerPoint Presentation ID Does Water Boil Hotter At Sea Level At sea level, water boils at 100 °c (212 °f) and freezes at 0 °c (32 °f). As we move higher into the atmosphere and the atmospheric pressure drops, so too does the amount of vapour pressure required for a liquid to boil. However, on mount everest at an altitude of. The answer is the water reaches its boiling point. Does Water Boil Hotter At Sea Level.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation Does Water Boil Hotter At Sea Level At sea level at a pressure of 1.013 bar, water begins to boil at a temperature of 100 °c. If you were to boil water that was pressurized greater than sea level atmospheric pressure, then yes, it can get hotter than 100 °c. As we move higher into the atmosphere and the atmospheric pressure drops, so too does the amount. Does Water Boil Hotter At Sea Level.
From www.youtube.com
Does Water Really Boil in a Vacuum Chamber? And Why? YouTube Does Water Boil Hotter At Sea Level If you were to boil water that was pressurized greater than sea level atmospheric pressure, then yes, it can get hotter than 100 °c. However, on mount everest at an altitude of. The answer is the water reaches its boiling point temperature and stays there. When water is heated to the boiling point, it reaches 100°c at sea level, and. Does Water Boil Hotter At Sea Level.
From www.weforum.org
This chart shows the oceans are getting warmer World Does Water Boil Hotter At Sea Level At sea level at a pressure of 1.013 bar, water begins to boil at a temperature of 100 °c. At sea level, water boils at 100 °c (212 °f) and freezes at 0 °c (32 °f). When water is heated to the boiling point, it reaches 100°c at sea level, and it transforms from a liquid to a gas, which. Does Water Boil Hotter At Sea Level.
From www.neuronmagazine.com
What Temperature Does Water Boil At 101 Does Water Boil Hotter At Sea Level Boiling point depends on pressure. The answer is the water reaches its boiling point temperature and stays there. As we move higher into the atmosphere and the atmospheric pressure drops, so too does the amount of vapour pressure required for a liquid to boil. However, on mount everest at an altitude of. Using this water boiling point calculator, boiling point. Does Water Boil Hotter At Sea Level.
From fyozkayiz.blob.core.windows.net
Why Does Water Boil At 100 Degrees Celsius At Sea Level at Jesus Newman Does Water Boil Hotter At Sea Level At sea level at a pressure of 1.013 bar, water begins to boil at a temperature of 100 °c. However, on mount everest at an altitude of. When water is heated to the boiling point, it reaches 100°c at sea level, and it transforms from a liquid to a gas, which we. At sea level, vapour pressure is equal to. Does Water Boil Hotter At Sea Level.
From www.myopencountry.com
Boiling Water at Higher Altitude What You Need to Know My Open Country Does Water Boil Hotter At Sea Level The temperature at which water boils isn’t the same everywhere. The answer is the water reaches its boiling point temperature and stays there. Using this water boiling point calculator, boiling point at sea level (1 atm or 29.92 inches of hg) is approximately 212 °f whereas at a height of. Boiling point depends on pressure. As we move higher into. Does Water Boil Hotter At Sea Level.
From aweseas.blogspot.com
Boiling Point Of Water Sea Level Does Water Boil Hotter At Sea Level When water is heated to the boiling point, it reaches 100°c at sea level, and it transforms from a liquid to a gas, which we. The answer is the water reaches its boiling point temperature and stays there. Boiling point depends on pressure. As we move higher into the atmosphere and the atmospheric pressure drops, so too does the amount. Does Water Boil Hotter At Sea Level.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level In Celsius Does Water Boil Hotter At Sea Level Using this water boiling point calculator, boiling point at sea level (1 atm or 29.92 inches of hg) is approximately 212 °f whereas at a height of. At sea level, water boils at 100 °c (212 °f) and freezes at 0 °c (32 °f). The answer is the water reaches its boiling point temperature and stays there. As we move. Does Water Boil Hotter At Sea Level.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level In Kelvin Does Water Boil Hotter At Sea Level At sea level, vapour pressure is equal to the atmospheric pressure at 100 ˚c, and so this is the temperature at which water boils. However, on mount everest at an altitude of. At sea level, water boils at 100 °c (212 °f) and freezes at 0 °c (32 °f). Using this water boiling point calculator, boiling point at sea level. Does Water Boil Hotter At Sea Level.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level Does Water Boil Hotter At Sea Level At sea level, vapour pressure is equal to the atmospheric pressure at 100 ˚c, and so this is the temperature at which water boils. Boiling point depends on pressure. If you were to boil water that was pressurized greater than sea level atmospheric pressure, then yes, it can get hotter than 100 °c. The answer is the water reaches its. Does Water Boil Hotter At Sea Level.
From chefd.com
What Temperature Does Water Boil? Chefd Does Water Boil Hotter At Sea Level At sea level, water boils at 100 °c (212 °f) and freezes at 0 °c (32 °f). The temperature at which water boils isn’t the same everywhere. At sea level, vapour pressure is equal to the atmospheric pressure at 100 ˚c, and so this is the temperature at which water boils. Using this water boiling point calculator, boiling point at. Does Water Boil Hotter At Sea Level.
From www.climatecentral.org
Ocean Heatwaves Climate Central Does Water Boil Hotter At Sea Level At sea level at a pressure of 1.013 bar, water begins to boil at a temperature of 100 °c. As we move higher into the atmosphere and the atmospheric pressure drops, so too does the amount of vapour pressure required for a liquid to boil. The answer is the water reaches its boiling point temperature and stays there. However, on. Does Water Boil Hotter At Sea Level.
From sciencenotes.org
How to Boil Water at Room Temperature Does Water Boil Hotter At Sea Level Using this water boiling point calculator, boiling point at sea level (1 atm or 29.92 inches of hg) is approximately 212 °f whereas at a height of. As we move higher into the atmosphere and the atmospheric pressure drops, so too does the amount of vapour pressure required for a liquid to boil. If you were to boil water that. Does Water Boil Hotter At Sea Level.
From www.youtube.com
What Happens When You Boil The Ocean? How To Make Homemade Sea Salt Does Water Boil Hotter At Sea Level Boiling point depends on pressure. At sea level, water boils at 100 °c (212 °f) and freezes at 0 °c (32 °f). As we move higher into the atmosphere and the atmospheric pressure drops, so too does the amount of vapour pressure required for a liquid to boil. However, on mount everest at an altitude of. The answer is the. Does Water Boil Hotter At Sea Level.
From fyozkayiz.blob.core.windows.net
Why Does Water Boil At 100 Degrees Celsius At Sea Level at Jesus Newman Does Water Boil Hotter At Sea Level However, on mount everest at an altitude of. The answer is the water reaches its boiling point temperature and stays there. Using this water boiling point calculator, boiling point at sea level (1 atm or 29.92 inches of hg) is approximately 212 °f whereas at a height of. If you were to boil water that was pressurized greater than sea. Does Water Boil Hotter At Sea Level.
From grizly.com
At sea level, what temperature does water boil? QuizGriz Does Water Boil Hotter At Sea Level If you were to boil water that was pressurized greater than sea level atmospheric pressure, then yes, it can get hotter than 100 °c. The answer is the water reaches its boiling point temperature and stays there. Using this water boiling point calculator, boiling point at sea level (1 atm or 29.92 inches of hg) is approximately 212 °f whereas. Does Water Boil Hotter At Sea Level.
From www3.epa.gov
Climate Impacts on Water Resources Climate Change US EPA Does Water Boil Hotter At Sea Level At sea level at a pressure of 1.013 bar, water begins to boil at a temperature of 100 °c. The answer is the water reaches its boiling point temperature and stays there. At sea level, vapour pressure is equal to the atmospheric pressure at 100 ˚c, and so this is the temperature at which water boils. The temperature at which. Does Water Boil Hotter At Sea Level.
From www.pdfprof.com
average boiling point of seawater Does Water Boil Hotter At Sea Level Boiling point depends on pressure. However, on mount everest at an altitude of. At sea level, vapour pressure is equal to the atmospheric pressure at 100 ˚c, and so this is the temperature at which water boils. As we move higher into the atmosphere and the atmospheric pressure drops, so too does the amount of vapour pressure required for a. Does Water Boil Hotter At Sea Level.
From www.neuronmagazine.com
What Temperature Does Water Boil At 101 Does Water Boil Hotter At Sea Level The temperature at which water boils isn’t the same everywhere. If you were to boil water that was pressurized greater than sea level atmospheric pressure, then yes, it can get hotter than 100 °c. Using this water boiling point calculator, boiling point at sea level (1 atm or 29.92 inches of hg) is approximately 212 °f whereas at a height. Does Water Boil Hotter At Sea Level.
From www.tec-science.com
Why does water boil faster at high altitudes? tecscience Does Water Boil Hotter At Sea Level At sea level at a pressure of 1.013 bar, water begins to boil at a temperature of 100 °c. As we move higher into the atmosphere and the atmospheric pressure drops, so too does the amount of vapour pressure required for a liquid to boil. Boiling point depends on pressure. At sea level, water boils at 100 °c (212 °f). Does Water Boil Hotter At Sea Level.
From waterfilterguru.com
At What Temperature Does Water Boil? Does Water Boil Hotter At Sea Level The temperature at which water boils isn’t the same everywhere. Using this water boiling point calculator, boiling point at sea level (1 atm or 29.92 inches of hg) is approximately 212 °f whereas at a height of. At sea level, vapour pressure is equal to the atmospheric pressure at 100 ˚c, and so this is the temperature at which water. Does Water Boil Hotter At Sea Level.
From bceweb.org
Water Boiling Chart A Visual Reference of Charts Chart Master Does Water Boil Hotter At Sea Level When water is heated to the boiling point, it reaches 100°c at sea level, and it transforms from a liquid to a gas, which we. The answer is the water reaches its boiling point temperature and stays there. As we move higher into the atmosphere and the atmospheric pressure drops, so too does the amount of vapour pressure required for. Does Water Boil Hotter At Sea Level.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level In Kelvin Does Water Boil Hotter At Sea Level The temperature at which water boils isn’t the same everywhere. Boiling point depends on pressure. At sea level, vapour pressure is equal to the atmospheric pressure at 100 ˚c, and so this is the temperature at which water boils. When water is heated to the boiling point, it reaches 100°c at sea level, and it transforms from a liquid to. Does Water Boil Hotter At Sea Level.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? Does Water Boil Hotter At Sea Level If you were to boil water that was pressurized greater than sea level atmospheric pressure, then yes, it can get hotter than 100 °c. When water is heated to the boiling point, it reaches 100°c at sea level, and it transforms from a liquid to a gas, which we. At sea level at a pressure of 1.013 bar, water begins. Does Water Boil Hotter At Sea Level.