Bromine Ion Formation . A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. Atoms of group 17 gain one electron and form anions with a 1− charge; A proton is removed from this intermediate, yielding a substituted benzene ring Large amounts of bromide salts are toxic from the action of soluble bromide ions, causing bromism. However, bromine is beneficial for human. Atoms of group 16 gain two electrons and form ions with a 2− charge,. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The enhanced mineralization of bpb during eo can be attributed to the formation of reactive bromine species, especially oxobromate i (bro ‒) which significantly participated. Formation of the strongly electrophilic bromine cation.
from periodictableguide.com
Large amounts of bromide salts are toxic from the action of soluble bromide ions, causing bromism. Formation of the strongly electrophilic bromine cation. Atoms of group 17 gain one electron and form anions with a 1− charge; A proton is removed from this intermediate, yielding a substituted benzene ring A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. Atoms of group 16 gain two electrons and form ions with a 2− charge,. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. However, bromine is beneficial for human. The enhanced mineralization of bpb during eo can be attributed to the formation of reactive bromine species, especially oxobromate i (bro ‒) which significantly participated.
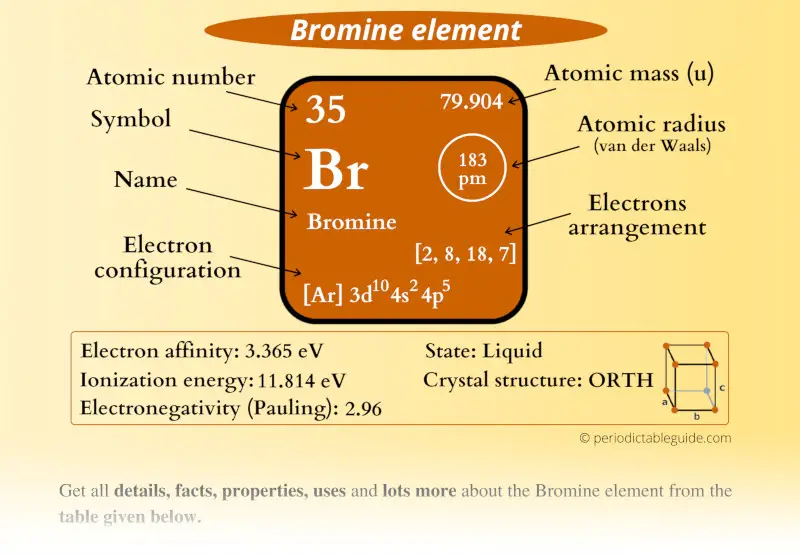
Bromine (Br) Periodic Table (Element Information & More)
Bromine Ion Formation Atoms of group 16 gain two electrons and form ions with a 2− charge,. Formation of the strongly electrophilic bromine cation. A proton is removed from this intermediate, yielding a substituted benzene ring However, bromine is beneficial for human. A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Large amounts of bromide salts are toxic from the action of soluble bromide ions, causing bromism. Atoms of group 16 gain two electrons and form ions with a 2− charge,. Atoms of group 17 gain one electron and form anions with a 1− charge; The enhanced mineralization of bpb during eo can be attributed to the formation of reactive bromine species, especially oxobromate i (bro ‒) which significantly participated.
From exycbirkc.blob.core.windows.net
Is Bromine An Atom Or Ion at Nancy Dunmire blog Bromine Ion Formation Atoms of group 16 gain two electrons and form ions with a 2− charge,. Large amounts of bromide salts are toxic from the action of soluble bromide ions, causing bromism. Formation of the strongly electrophilic bromine cation. A proton is removed from this intermediate, yielding a substituted benzene ring However, bromine is beneficial for human. Alkenes react in the cold. Bromine Ion Formation.
From www.alamy.com
Bromine molecule, illustration Stock Photo Alamy Bromine Ion Formation The enhanced mineralization of bpb during eo can be attributed to the formation of reactive bromine species, especially oxobromate i (bro ‒) which significantly participated. Atoms of group 16 gain two electrons and form ions with a 2− charge,. Formation of the strongly electrophilic bromine cation. However, bromine is beneficial for human. Large amounts of bromide salts are toxic from. Bromine Ion Formation.
From www.masterorganicchemistry.com
Bromination of Alkenes The Mechanism Master Organic Chemistry Bromine Ion Formation Atoms of group 16 gain two electrons and form ions with a 2− charge,. Large amounts of bromide salts are toxic from the action of soluble bromide ions, causing bromism. The enhanced mineralization of bpb during eo can be attributed to the formation of reactive bromine species, especially oxobromate i (bro ‒) which significantly participated. A proton is removed from. Bromine Ion Formation.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine Ion Formation The enhanced mineralization of bpb during eo can be attributed to the formation of reactive bromine species, especially oxobromate i (bro ‒) which significantly participated. Formation of the strongly electrophilic bromine cation. However, bromine is beneficial for human. A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble. Bromine Ion Formation.
From blog.orendatech.com
Understanding Bromine Pools and Spas Bromine Ion Formation A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. The enhanced mineralization of bpb during eo can be attributed to the formation of reactive bromine species, especially oxobromate i (bro ‒) which significantly participated. Formation of the strongly electrophilic bromine cation. Atoms of group 17 gain. Bromine Ion Formation.
From www.numerade.com
draw models to represent the formation of the positive calcium ion and Bromine Ion Formation The enhanced mineralization of bpb during eo can be attributed to the formation of reactive bromine species, especially oxobromate i (bro ‒) which significantly participated. However, bromine is beneficial for human. Atoms of group 17 gain one electron and form anions with a 1− charge; Alkenes react in the cold with pure liquid bromine, or with a solution of bromine. Bromine Ion Formation.
From studiousguy.com
Bromine (Br) Properties & Uses StudiousGuy Bromine Ion Formation Formation of the strongly electrophilic bromine cation. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. However, bromine is beneficial for human. A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. A proton is. Bromine Ion Formation.
From www.istockphoto.com
Br Bromine Element Information Facts Properties Trends Uses And Bromine Ion Formation Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. Large amounts of bromide salts are toxic from the action of soluble bromide ions, causing bromism.. Bromine Ion Formation.
From sciencenotes.org
Bromine Facts Atomic Number 35 and Element Symbol Br Bromine Ion Formation Large amounts of bromide salts are toxic from the action of soluble bromide ions, causing bromism. A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. Atoms of group 16 gain two electrons and form ions with a 2− charge,. Formation of the strongly electrophilic bromine cation.. Bromine Ion Formation.
From www.youtube.com
Bromonium ion formation Alkyl Halide Synthesis YouTube Bromine Ion Formation A proton is removed from this intermediate, yielding a substituted benzene ring Large amounts of bromide salts are toxic from the action of soluble bromide ions, causing bromism. Atoms of group 17 gain one electron and form anions with a 1− charge; The enhanced mineralization of bpb during eo can be attributed to the formation of reactive bromine species, especially. Bromine Ion Formation.
From www.youtube.com
Quick video Balancing an oxidation reduction reaction in base [bromine Bromine Ion Formation Atoms of group 16 gain two electrons and form ions with a 2− charge,. A proton is removed from this intermediate, yielding a substituted benzene ring Atoms of group 17 gain one electron and form anions with a 1− charge; A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as. Bromine Ion Formation.
From www.pinterest.com
Bromine Facts Atomic Number 35 and Element Symbol Br Bromine Ion Formation Atoms of group 16 gain two electrons and form ions with a 2− charge,. A proton is removed from this intermediate, yielding a substituted benzene ring The enhanced mineralization of bpb during eo can be attributed to the formation of reactive bromine species, especially oxobromate i (bro ‒) which significantly participated. Atoms of group 17 gain one electron and form. Bromine Ion Formation.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Bromine Ion Formation However, bromine is beneficial for human. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The enhanced mineralization of bpb during eo can be attributed to the formation of reactive bromine species, especially oxobromate i (bro ‒) which significantly participated. Large amounts of bromide salts are toxic from. Bromine Ion Formation.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Bromine Ion Formation Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. However, bromine is beneficial for human. Large amounts of bromide salts are toxic from the action of soluble bromide ions, causing bromism. Atoms of group 16 gain two electrons and form ions with a 2− charge,. Formation of the. Bromine Ion Formation.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Ion Formation Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms of group 16 gain two electrons and form ions with a 2− charge,. However, bromine is beneficial for human. Large amounts of bromide salts are toxic from the action of soluble bromide ions, causing bromism. A proton is removed from this intermediate, yielding a substituted. Bromine Ion Formation.
From periodictableguide.com
Bromine (Br) Periodic Table (Element Information & More) Bromine Ion Formation The enhanced mineralization of bpb during eo can be attributed to the formation of reactive bromine species, especially oxobromate i (bro ‒) which significantly participated. However, bromine is beneficial for human. Formation of the strongly electrophilic bromine cation. A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble. Bromine Ion Formation.
From www.britannica.com
Bromine Properties, Uses, & Facts Britannica Bromine Ion Formation Large amounts of bromide salts are toxic from the action of soluble bromide ions, causing bromism. However, bromine is beneficial for human. The enhanced mineralization of bpb during eo can be attributed to the formation of reactive bromine species, especially oxobromate i (bro ‒) which significantly participated. A bromine ion, denoted as br −, is the common negative univalent form. Bromine Ion Formation.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Bromine Ion Formation A proton is removed from this intermediate, yielding a substituted benzene ring Large amounts of bromide salts are toxic from the action of soluble bromide ions, causing bromism. Atoms of group 16 gain two electrons and form ions with a 2− charge,. However, bromine is beneficial for human. A bromine ion, denoted as br −, is the common negative univalent. Bromine Ion Formation.
From www.slideserve.com
PPT Chapter 8 Alkenes Reactions and Synthesis PowerPoint Bromine Ion Formation Atoms of group 17 gain one electron and form anions with a 1− charge; A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Atoms of. Bromine Ion Formation.
From slidetodoc.com
Compounds Molecules Molecule The smallest identifiable unit that Bromine Ion Formation Atoms of group 16 gain two electrons and form ions with a 2− charge,. The enhanced mineralization of bpb during eo can be attributed to the formation of reactive bromine species, especially oxobromate i (bro ‒) which significantly participated. A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water. Bromine Ion Formation.
From www.youtube.com
Br Electron Configuration (Bromide Ion) YouTube Bromine Ion Formation A proton is removed from this intermediate, yielding a substituted benzene ring A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. The enhanced mineralization of bpb during eo can be attributed to the formation of reactive bromine species, especially oxobromate i (bro ‒) which significantly participated.. Bromine Ion Formation.
From mungfali.com
Aufbau Diagram For Bromine Bromine Ion Formation The enhanced mineralization of bpb during eo can be attributed to the formation of reactive bromine species, especially oxobromate i (bro ‒) which significantly participated. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. However, bromine is beneficial for human. Atoms of group 16 gain two electrons and. Bromine Ion Formation.
From giofdhyrk.blob.core.windows.net
When Bromine An Ion What Charge And Name Does It Have at Faye Bromine Ion Formation Atoms of group 16 gain two electrons and form ions with a 2− charge,. Formation of the strongly electrophilic bromine cation. A proton is removed from this intermediate, yielding a substituted benzene ring However, bromine is beneficial for human. Large amounts of bromide salts are toxic from the action of soluble bromide ions, causing bromism. A bromine ion, denoted as. Bromine Ion Formation.
From in.pinterest.com
Bromine Element Information, Facts, Properties, Trends,Uses Bromine Ion Formation Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Formation of the strongly electrophilic bromine cation. Large amounts of bromide salts are toxic from the action of soluble bromide ions, causing bromism. The enhanced mineralization of bpb during eo can be attributed to the formation of reactive bromine. Bromine Ion Formation.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of Bromine Ion Formation A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. A proton is removed from this intermediate, yielding a substituted benzene ring However, bromine is beneficial for human. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent. Bromine Ion Formation.
From www.researchgate.net
(a) Simplified scheme showing the formation of reactive bromine species Bromine Ion Formation Formation of the strongly electrophilic bromine cation. The enhanced mineralization of bpb during eo can be attributed to the formation of reactive bromine species, especially oxobromate i (bro ‒) which significantly participated. A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. Atoms of group 16 gain. Bromine Ion Formation.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine Ion Formation Atoms of group 17 gain one electron and form anions with a 1− charge; Large amounts of bromide salts are toxic from the action of soluble bromide ions, causing bromism. However, bromine is beneficial for human. A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. Formation. Bromine Ion Formation.
From ar.inspiredpencil.com
Atomic Structure Of Bromine Bromine Ion Formation A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. Formation of the strongly electrophilic bromine cation. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The enhanced mineralization of bpb during eo can be. Bromine Ion Formation.
From www.youtube.com
Ionic Charge for Bromine (Br) YouTube Bromine Ion Formation The enhanced mineralization of bpb during eo can be attributed to the formation of reactive bromine species, especially oxobromate i (bro ‒) which significantly participated. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Large amounts of bromide salts are toxic from the action of soluble bromide ions,. Bromine Ion Formation.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Bromine Ion Formation Atoms of group 16 gain two electrons and form ions with a 2− charge,. Large amounts of bromide salts are toxic from the action of soluble bromide ions, causing bromism. A proton is removed from this intermediate, yielding a substituted benzene ring Atoms of group 17 gain one electron and form anions with a 1− charge; The enhanced mineralization of. Bromine Ion Formation.
From www.researchgate.net
a Formation of a bromine molecule from dissociative adsorption of two Bromine Ion Formation Formation of the strongly electrophilic bromine cation. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The enhanced mineralization of bpb during eo can be attributed to the formation of reactive bromine species, especially oxobromate i (bro ‒) which significantly participated. Atoms of group 17 gain one electron. Bromine Ion Formation.
From www.alamy.com
Symbol and electron diagram for Bromine Stock Vector Image & Art Alamy Bromine Ion Formation Atoms of group 16 gain two electrons and form ions with a 2− charge,. A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. Large amounts of bromide salts are toxic from the action of soluble bromide ions, causing bromism. The enhanced mineralization of bpb during eo. Bromine Ion Formation.
From www.alamy.com
Chemist atom of Bromine diagram Stock Vector Image & Art Alamy Bromine Ion Formation Formation of the strongly electrophilic bromine cation. The enhanced mineralization of bpb during eo can be attributed to the formation of reactive bromine species, especially oxobromate i (bro ‒) which significantly participated. However, bromine is beneficial for human. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Atoms. Bromine Ion Formation.
From cepvgsav.blob.core.windows.net
Bromine Molecule Lewis Structure at Therese Boyd blog Bromine Ion Formation The enhanced mineralization of bpb during eo can be attributed to the formation of reactive bromine species, especially oxobromate i (bro ‒) which significantly participated. Atoms of group 17 gain one electron and form anions with a 1− charge; Large amounts of bromide salts are toxic from the action of soluble bromide ions, causing bromism. A bromine ion, denoted as. Bromine Ion Formation.
From studiousguy.com
Bromine (Br) Properties & Uses StudiousGuy Bromine Ion Formation Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Formation of the strongly electrophilic bromine cation. However, bromine is beneficial for human. A proton is removed from this intermediate, yielding a substituted benzene ring Large amounts of bromide salts are toxic from the action of soluble bromide ions,. Bromine Ion Formation.