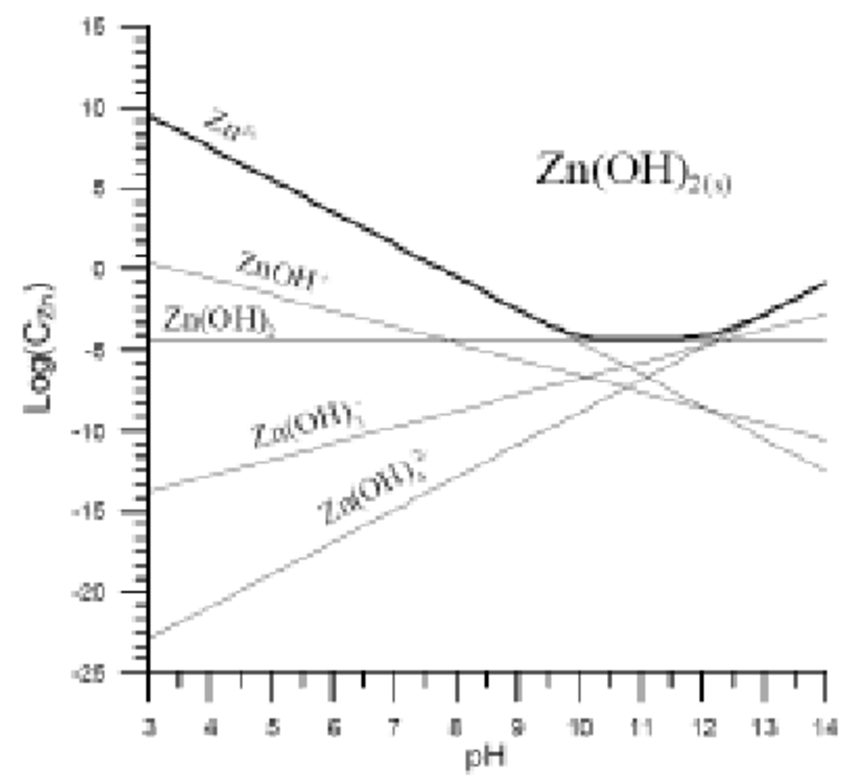
Zinc Chloride Water Solubility . Zinc chlorides, of which at nine crystalline forms are known, are colorless or white and highly soluble in water. Zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. It is soluble in water. It is used for preserving wood, in soldering fluxes, as a catalyst. Acidify the solution by addition. Water temperature can have a significant effect on the solubility of compounds. To make a zncl2 solution (even at low molarity), add water to zncl2. It is corrosive to metals and therefore irritating to the skin, eyes and mucous membranes. Refer to the chart below to find reference values per gram of. All of them are highly soluble in water. Zinc chloride exhibits hygroscopic qualities, i.e. It attracts and captures the water molecules from the environment. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the solubility constant, to. At this point, a white precipitate will form corresponding to zn oxychloride. Therefore it is very essential to protect zinc chloride from moisture.
from mavink.com
Acidify the solution by addition. Refer to the chart below to find reference values per gram of. It attracts and captures the water molecules from the environment. All of them are highly soluble in water. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the solubility constant, to. Zinc chloride exhibits hygroscopic qualities, i.e. To make a zncl2 solution (even at low molarity), add water to zncl2. Zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. Zncl 2 itself is hygroscopic. It is corrosive to metals and therefore irritating to the skin, eyes and mucous membranes.
Metal Solubility Chart
Zinc Chloride Water Solubility It is corrosive to metals and therefore irritating to the skin, eyes and mucous membranes. It is used for preserving wood, in soldering fluxes, as a catalyst. Therefore it is very essential to protect zinc chloride from moisture. To make a zncl2 solution (even at low molarity), add water to zncl2. It is soluble in water. All of them are highly soluble in water. It is corrosive to metals and therefore irritating to the skin, eyes and mucous membranes. At this point, a white precipitate will form corresponding to zn oxychloride. Zinc chloride exhibits hygroscopic qualities, i.e. Water temperature can have a significant effect on the solubility of compounds. Zncl 2 itself is hygroscopic. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the solubility constant, to. It attracts and captures the water molecules from the environment. Refer to the chart below to find reference values per gram of. Zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. Zinc chlorides, of which at nine crystalline forms are known, are colorless or white and highly soluble in water.
From www.youtube.com
Equation for ZnCl2 + H2O (Zinc chloride + Water) YouTube Zinc Chloride Water Solubility Therefore it is very essential to protect zinc chloride from moisture. All of them are highly soluble in water. It attracts and captures the water molecules from the environment. Acidify the solution by addition. It is used for preserving wood, in soldering fluxes, as a catalyst. Zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid. Zinc Chloride Water Solubility.
From www.reddit.com
Spontaneous Reactions and solids that will dissolve in acids r/chemhelp Zinc Chloride Water Solubility It is soluble in water. Zinc chlorides, of which at nine crystalline forms are known, are colorless or white and highly soluble in water. It is corrosive to metals and therefore irritating to the skin, eyes and mucous membranes. All of them are highly soluble in water. It attracts and captures the water molecules from the environment. It is used. Zinc Chloride Water Solubility.
From www.scribd.com
Organic_solvents Data With Water Solubility Solvent Functional Group Zinc Chloride Water Solubility It is used for preserving wood, in soldering fluxes, as a catalyst. It is soluble in water. Zinc chlorides, of which at nine crystalline forms are known, are colorless or white and highly soluble in water. Zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. It attracts and captures. Zinc Chloride Water Solubility.
From wou.edu
CH150 Chapter 7 Solutions Chemistry Zinc Chloride Water Solubility It attracts and captures the water molecules from the environment. It is corrosive to metals and therefore irritating to the skin, eyes and mucous membranes. It is used for preserving wood, in soldering fluxes, as a catalyst. At this point, a white precipitate will form corresponding to zn oxychloride. All of them are highly soluble in water. Zinc chloride, represented. Zinc Chloride Water Solubility.
From www.slideserve.com
PPT Reactions in Solution PowerPoint Presentation, free download ID Zinc Chloride Water Solubility Therefore it is very essential to protect zinc chloride from moisture. It attracts and captures the water molecules from the environment. Refer to the chart below to find reference values per gram of. Zncl 2 itself is hygroscopic. Zinc chloride exhibits hygroscopic qualities, i.e. It is soluble in water. Zinc chlorides, of which at nine crystalline forms are known, are. Zinc Chloride Water Solubility.
From www.youtube.com
Is ZnCl2 Soluble or Insoluble in Water? YouTube Zinc Chloride Water Solubility Zinc chlorides, of which at nine crystalline forms are known, are colorless or white and highly soluble in water. Zncl 2 itself is hygroscopic. Zinc chloride exhibits hygroscopic qualities, i.e. Acidify the solution by addition. Refer to the chart below to find reference values per gram of. Therefore it is very essential to protect zinc chloride from moisture. At this. Zinc Chloride Water Solubility.
From galvanizeit.org
In Chemical Solutions American Galvanizers Association Zinc Chloride Water Solubility Acidify the solution by addition. At this point, a white precipitate will form corresponding to zn oxychloride. It is used for preserving wood, in soldering fluxes, as a catalyst. It is soluble in water. Refer to the chart below to find reference values per gram of. Zinc chlorides, of which at nine crystalline forms are known, are colorless or white. Zinc Chloride Water Solubility.
From slideplayer.com
Cysistrollee. ppt download Zinc Chloride Water Solubility Water temperature can have a significant effect on the solubility of compounds. To make a zncl2 solution (even at low molarity), add water to zncl2. Zinc chloride exhibits hygroscopic qualities, i.e. It attracts and captures the water molecules from the environment. Therefore it is very essential to protect zinc chloride from moisture. Zinc chloride, represented by the chemical formula zncl. Zinc Chloride Water Solubility.
From gionioblo.blob.core.windows.net
Zinc Chloride Metal Equation at Randy Edwards blog Zinc Chloride Water Solubility Zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. All of them are highly soluble in water. Refer to the chart below to find reference values per gram of. Therefore it is very essential to protect zinc chloride from moisture. It is used for preserving wood, in soldering fluxes,. Zinc Chloride Water Solubility.
From www.researchgate.net
What is the proper way to prepare a zinc chloride containing buffer for Zinc Chloride Water Solubility It is used for preserving wood, in soldering fluxes, as a catalyst. It is corrosive to metals and therefore irritating to the skin, eyes and mucous membranes. Water temperature can have a significant effect on the solubility of compounds. Therefore it is very essential to protect zinc chloride from moisture. It attracts and captures the water molecules from the environment.. Zinc Chloride Water Solubility.
From www.altichem.com
Altichem Zinc Chloride Water Solubility All of them are highly soluble in water. Zinc chloride exhibits hygroscopic qualities, i.e. Water temperature can have a significant effect on the solubility of compounds. It attracts and captures the water molecules from the environment. Refer to the chart below to find reference values per gram of. Therefore it is very essential to protect zinc chloride from moisture. Zinc. Zinc Chloride Water Solubility.
From studymind.co.uk
ᐉ Solubility Rules Insoluble & Soluble Salts Making Zinc Chloride Water Solubility In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the solubility constant, to. It is soluble in water. At this point, a white precipitate will form corresponding to zn oxychloride. It is used for preserving wood, in soldering fluxes, as a catalyst. It is corrosive to metals and therefore. Zinc Chloride Water Solubility.
From www.slideserve.com
PPT Terrence P. Sherlock Burlington County College 2004 PowerPoint Zinc Chloride Water Solubility All of them are highly soluble in water. At this point, a white precipitate will form corresponding to zn oxychloride. Zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. It is corrosive to metals and therefore irritating to the skin, eyes and mucous membranes. It is used for preserving. Zinc Chloride Water Solubility.
From www.semanticscholar.org
Figure 1 from COMPARISON OF SOLUBILITY OF ZINC PHOSPHATE AND GLASS Zinc Chloride Water Solubility In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the solubility constant, to. It is corrosive to metals and therefore irritating to the skin, eyes and mucous membranes. Water temperature can have a significant effect on the solubility of compounds. Zncl 2 itself is hygroscopic. At this point, a. Zinc Chloride Water Solubility.
From www.chemizoenterprise.in
Fertilizers Water Soluble Fertilizer Wholesale Trader from Ahmedabad Zinc Chloride Water Solubility It is corrosive to metals and therefore irritating to the skin, eyes and mucous membranes. Refer to the chart below to find reference values per gram of. All of them are highly soluble in water. It is soluble in water. Therefore it is very essential to protect zinc chloride from moisture. At this point, a white precipitate will form corresponding. Zinc Chloride Water Solubility.
From www.triangulumchemicals.in
Zinc Chloride Manufacturer,Supplier,Exporter Zinc Chloride Water Solubility In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the solubility constant, to. Zinc chloride exhibits hygroscopic qualities, i.e. Zinc chlorides, of which at nine crystalline forms are known, are colorless or white and highly soluble in water. It attracts and captures the water molecules from the environment. Acidify. Zinc Chloride Water Solubility.
From gionioblo.blob.core.windows.net
Zinc Chloride Metal Equation at Randy Edwards blog Zinc Chloride Water Solubility Therefore it is very essential to protect zinc chloride from moisture. At this point, a white precipitate will form corresponding to zn oxychloride. It is soluble in water. Acidify the solution by addition. Zncl 2 itself is hygroscopic. Zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. It attracts. Zinc Chloride Water Solubility.
From chemistry.stackexchange.com
Why does sodium sulfate have an unusual solubilitytemperature curve Zinc Chloride Water Solubility Zinc chloride exhibits hygroscopic qualities, i.e. Acidify the solution by addition. Water temperature can have a significant effect on the solubility of compounds. It is used for preserving wood, in soldering fluxes, as a catalyst. Zncl 2 itself is hygroscopic. Zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water.. Zinc Chloride Water Solubility.
From www.indiamart.com
Zinc Chloride, 25 Kg Bag at best price in Mumbai ID 26287348733 Zinc Chloride Water Solubility It attracts and captures the water molecules from the environment. Zncl 2 itself is hygroscopic. To make a zncl2 solution (even at low molarity), add water to zncl2. It is soluble in water. Water temperature can have a significant effect on the solubility of compounds. Therefore it is very essential to protect zinc chloride from moisture. Acidify the solution by. Zinc Chloride Water Solubility.
From www.chemizoenterprise.in
Fertilizers Water Soluble Fertilizer Wholesale Trader from Ahmedabad Zinc Chloride Water Solubility Water temperature can have a significant effect on the solubility of compounds. Zinc chloride exhibits hygroscopic qualities, i.e. At this point, a white precipitate will form corresponding to zn oxychloride. To make a zncl2 solution (even at low molarity), add water to zncl2. Therefore it is very essential to protect zinc chloride from moisture. All of them are highly soluble. Zinc Chloride Water Solubility.
From www.indiamart.com
Zinc Chloride Pure at Rs 130/kg Zinc Chloride in Raigad ID Zinc Chloride Water Solubility It is corrosive to metals and therefore irritating to the skin, eyes and mucous membranes. Therefore it is very essential to protect zinc chloride from moisture. It is used for preserving wood, in soldering fluxes, as a catalyst. Zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. At this. Zinc Chloride Water Solubility.
From www.youtube.com
Double displacement of ZnSO4 + BaCl2 Zinc sulphate + Barium chloride Zinc Chloride Water Solubility In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the solubility constant, to. It is corrosive to metals and therefore irritating to the skin, eyes and mucous membranes. Zinc chlorides, of which at nine crystalline forms are known, are colorless or white and highly soluble in water. It attracts. Zinc Chloride Water Solubility.
From projectreports.eiriindia.org
ZINC CHLORIDE (ZnCl2) Project Report Manufacturing Process Books Zinc Chloride Water Solubility It attracts and captures the water molecules from the environment. It is soluble in water. It is corrosive to metals and therefore irritating to the skin, eyes and mucous membranes. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the solubility constant, to. Therefore it is very essential to. Zinc Chloride Water Solubility.
From www.dolchem.com
Zinc Chloride DOLCHEM Zinc Chloride Water Solubility At this point, a white precipitate will form corresponding to zn oxychloride. Water temperature can have a significant effect on the solubility of compounds. Zncl 2 itself is hygroscopic. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the solubility constant, to. It is used for preserving wood, in. Zinc Chloride Water Solubility.
From calebcroomphysci4dummies.weebly.com
Solubility Physical Science For Dummies Zinc Chloride Water Solubility Zinc chlorides, of which at nine crystalline forms are known, are colorless or white and highly soluble in water. At this point, a white precipitate will form corresponding to zn oxychloride. Zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. Zncl 2 itself is hygroscopic. It is soluble in. Zinc Chloride Water Solubility.
From www.engineeringtoolbox.com
Density of aqueous solutions of chlorides Zinc Chloride Water Solubility At this point, a white precipitate will form corresponding to zn oxychloride. It is used for preserving wood, in soldering fluxes, as a catalyst. Zncl 2 itself is hygroscopic. To make a zncl2 solution (even at low molarity), add water to zncl2. Water temperature can have a significant effect on the solubility of compounds. Zinc chloride exhibits hygroscopic qualities, i.e.. Zinc Chloride Water Solubility.
From www.flinnsci.ca
Solubility Rules Charts for Chemistry Zinc Chloride Water Solubility Zinc chloride exhibits hygroscopic qualities, i.e. All of them are highly soluble in water. At this point, a white precipitate will form corresponding to zn oxychloride. It attracts and captures the water molecules from the environment. Refer to the chart below to find reference values per gram of. It is soluble in water. Zinc chlorides, of which at nine crystalline. Zinc Chloride Water Solubility.
From www.researchgate.net
Solubility curves for common metals in freshwater with pH [60 Zinc Chloride Water Solubility Zinc chloride exhibits hygroscopic qualities, i.e. It is corrosive to metals and therefore irritating to the skin, eyes and mucous membranes. Acidify the solution by addition. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the solubility constant, to. Zncl 2 itself is hygroscopic. Water temperature can have a. Zinc Chloride Water Solubility.
From mavink.com
Metal Solubility Chart Zinc Chloride Water Solubility It is used for preserving wood, in soldering fluxes, as a catalyst. Zinc chloride exhibits hygroscopic qualities, i.e. Zncl 2 itself is hygroscopic. Refer to the chart below to find reference values per gram of. Water temperature can have a significant effect on the solubility of compounds. It is soluble in water. All of them are highly soluble in water.. Zinc Chloride Water Solubility.
From depositphotos.com
Test Chloride Infographic Diagram Showing Laboratory Experiment Zinc Chloride Water Solubility It attracts and captures the water molecules from the environment. Therefore it is very essential to protect zinc chloride from moisture. To make a zncl2 solution (even at low molarity), add water to zncl2. It is corrosive to metals and therefore irritating to the skin, eyes and mucous membranes. All of them are highly soluble in water. Refer to the. Zinc Chloride Water Solubility.
From cymitquimica.com
Zinc Chloride (High Purity & Low water content) 3BZ0053 Zinc Chloride Water Solubility It attracts and captures the water molecules from the environment. It is corrosive to metals and therefore irritating to the skin, eyes and mucous membranes. Zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. Zinc chlorides, of which at nine crystalline forms are known, are colorless or white and. Zinc Chloride Water Solubility.
From www.shutterstock.com
Zinc Chloride Formula Zncl2 Cl2zn White ilustración de stock 769733368 Zinc Chloride Water Solubility Zncl 2 itself is hygroscopic. It attracts and captures the water molecules from the environment. Water temperature can have a significant effect on the solubility of compounds. Zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. It is soluble in water. In this section we will apply chemical equilibria. Zinc Chloride Water Solubility.
From www.linstitute.net
CIE A Level Chemistry复习笔记2.2.2 Reactions of Group 2 Oxides, Hydroxides Zinc Chloride Water Solubility All of them are highly soluble in water. At this point, a white precipitate will form corresponding to zn oxychloride. Zncl 2 itself is hygroscopic. Zinc chlorides, of which at nine crystalline forms are known, are colorless or white and highly soluble in water. Water temperature can have a significant effect on the solubility of compounds. Zinc chloride exhibits hygroscopic. Zinc Chloride Water Solubility.
From socratic.org
Substance A will not dissolve in water. What can be said about Zinc Chloride Water Solubility Zncl 2 itself is hygroscopic. Acidify the solution by addition. It is corrosive to metals and therefore irritating to the skin, eyes and mucous membranes. At this point, a white precipitate will form corresponding to zn oxychloride. Zinc chloride exhibits hygroscopic qualities, i.e. In this section we will apply chemical equilibria to the concept of solubility and introduce a type. Zinc Chloride Water Solubility.
From 2012books.lardbucket.org
Describing Electrochemical Cells Zinc Chloride Water Solubility Zinc chloride exhibits hygroscopic qualities, i.e. It is used for preserving wood, in soldering fluxes, as a catalyst. Refer to the chart below to find reference values per gram of. Therefore it is very essential to protect zinc chloride from moisture. It attracts and captures the water molecules from the environment. All of them are highly soluble in water. Water. Zinc Chloride Water Solubility.